Summary
An investigational anti-interferon-a monoclonal antibody, sifalimumab, reduced global disease activity in patients with systemic lupus erythematosus (SLE). This article presents results from a phase 2b study of sifalimumab in patients with moderate to severe SLE.
- Rheumatology Clinical Trials
- Lupus
- Rheumatology Clinical Trials
- Rheumatology
- Lupus
An investigational anti-interferon (IFN)-α monoclonal antibody, sifalimumab, reduced global disease activity in patients with systemic lupus erythematosus (SLE). Munther Khamashta, MD, St Thomas' Hospital, London, United Kingdom, presented results from a phase 2b study of sifalimumab in patients with moderate to severe SLE.
Type I IFNs play a key role in the pathogenesis of SLE [Crow MK. J Immunol. 2014; Elkon KB, Wiedman A. Curr Opin Rheumatol. 2012; Elkon KB, Stone V V. J Interferon Cytokine Res. 2011; Rönnblom L et al. Semin Immunol. 2011; Dall'era MC et al. Ann Rhuem Dis. 2005]. Type I IFNs activate multiple pathways central to SLE pathogenesis [Kirou KA et al. Arthritis Rheum. 2005], including activation of monocytes, dendritic cells, neutrophils, T cells, and B cells. IFN-α is the predominant subtype of type I IFNs [Hillyer P et al. Immunol Cell Biol. 2012]. Sifalimumab is a fully human monoclonal antibody binding and neutralizing the majority of IFN-α subtypes [Merrill JT et al. Ann Rheum Dis. 2011].
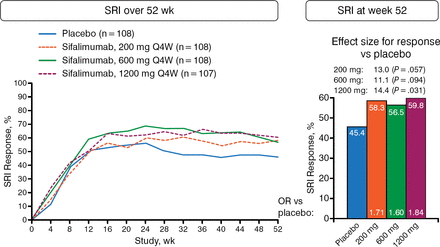
The study included 431 adults with moderate to severe SLE and with a SLE Disease Activity Index 2000 ≥ 6 and a Physician's Global Assessment ≥ 1.0 at screening. Patients were receiving standard-of-care treatment at the time that they were randomized to placebo or 1 of 3 monthly doses of sifalimumab administered intravenously: 200, 600, or 1200 mg. The primary end point was the percentage of patients that responded as measured by the Systemic Lupus Erythematosus Responder Index (SRI) at week 52. Approximately 85% of the patients in each randomized group completed the study.
The percentage of patients that achieved an SRI-4 response at week 52 was higher for sifalimumab at all doses vs placebo (Figure 1). The effect size for response vs placebo was 13.0% with 200 mg (P = .057), 11.1% with 600 mg (P = .094), and 14.4% with 1200 mg (P = .031).
Primary End Point: SRI Response
OR, odds ratio; Q4W, every 4 weeks; SRI, Systemic Lupus Erythematosus Responder Index.
Reproduced with permission from M Khamashta, MD.
The percentage of responders using the more stringent SRI-6 was also higher at all doses of sifalimumab (placebo, 37.4%; 200 mg, 50.0%; 600 mg, 43.5%; 1200 mg, 53.3%). The effect size for response vs placebo was 12.6% with 200 mg (P = .051), 6.1% with 600 mg (P = .301), and 15.9% with 1200 mg (P = .016).
The British Isles Lupus Assessment Group-based Combined Lupus Assessment response at week 52 was also higher in patients randomized to sifalimumab compared with placebo (placebo, 36.1%; 200 mg, 45.4%; 600 mg, 46.7%; 1200 mg, 48.1%).
A Cutaneous Lupus Erythematosus Disease Area and Severity Index response (≥ 4-point reduction from baseline) in patients with moderate to severe skin involvement was achieved by more patients randomized to sifalimumab compared with placebo (placebo, 48.6%; 200 mg, 72.7%; 600 mg, 57.6%; 1200 mg, 73.1%).
The number of patients with ≥ 8 swollen and ≥ 8 tender joints at baseline who achieved a ≥ 50% decrease in swollen and tender joint count was an exploratory end point. On this measure, the response rate is higher at all doses of sifalimumab (placebo, 36.8%; 200 mg, 53.7%; 600 mg, 57.9%; 1200 mg, 60.5%).
Response rates on the SRI-4 were higher than placebo with sifalimumab regardless of high or low IFN gene signatures.
Most commonly reported adverse events (AEs) were similar across groups, including worsening SLE (sifalimumab 30.0% vs placebo 34.3%), urinary tract infection (17.6% vs 13.9%), and headache (13.3% vs 13.9%). Serious AEs occurred in 18.3% of the sifalimumab group vs 17.6% of the placebo group. Herpes zoster infection was more common in sifalimumab recipients vs placebo (5.9% vs 0.9%).
The overall efficacy results suggest that 1200 mg monthly is the most efficacious dose of sifalimumab for use in moderate to severe SLE.
- © 2014 MD Conference Express®