Summary
A panel of 3 rheumatologists spoke of the challenges in developing and implementing the first-ever 2012 gout guidelines published in 2 parts by the American College of Rheumatology (ACR). Specific topics include an overview of the guideline development process, the new method adopted by the ACR to develop guidelines, as well as a discussion of the difficulties in implementing treatment guidelines.
- Exclusive Article - For home page
- Rheumatology Guidelines
- Inflammatory Disorders
- Exclusive Article - For home page
- Rheumatology Guidelines
- Inflammatory Disorders
- Rheumatology
Clinical guidelines are an integral part of health care quality. If widely disseminated to their target audience, guidelines should lead to continuous quality improvement and better patient outcomes. Aside from serving as a clinical roadmap, guidelines can also be used as benchmarks to develop quality measures. However, there are many challenges in choosing the best data to inform the guidelines and in directing the flow of the guidelines from the practitioner to the patient.
A panel of 3 rheumatologists spoke of the challenges in developing and implementing the first-ever 2012 gout guidelines published in 2 parts by the American College of Rheumatology (ACR) [Khanna D et al. Arthritis Care Res. 2012]. John D. FitzGerald, MD, PhD, University of California, Los Angeles (UCLA), Los Angeles, California, USA, a principal investigator on the guidelines committee, presented an overview of the guideline development process.
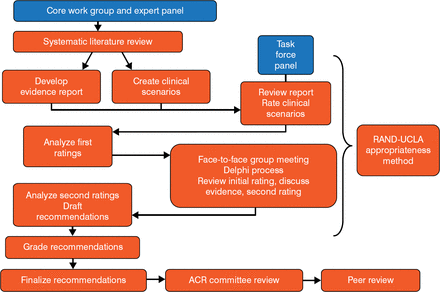
The guidelines were developed using the RAND/UCLA Appropriateness Method (Figure 1). Of note is that the RAND model does not include cost-effectiveness as part of its methodology, because there is typically a paucity of cost-effectiveness data to address the clinical questions posed by the guidelines.
ACR Clinical Guideline Development
ACR, American College of Rheumatology; UCLA, University of California, Los Angeles. Reproduced with permission from JD FitzGerald, MD, PhD.
The systematic literature review originally identified 5380 articles for consideration. After each article was subjected to a hierarchy of evidence, the literature was then whittled down to 51 manuscripts and 5 abstracts covering acute gout, urate-lowering therapy, and gout prophylaxis. Each manuscript or abstract was then graded as level A, B, or C (best to least desirable; Table 1) and was used to create hundreds of clinical case scenarios related to a particular process of care. Ideally, all guidelines would be based on the best possible evidence and tempered by clinical judgment. However, despite the best efforts of evidence teams, many guidelines are based on weaker, level C evidence.
Grading the Evidence
The Task Force then voted on the appropriateness of each case scenario using a Likert scale of 1 to 9. The results were then pooled and the median score calculated. Any score of at least 7 advanced to a treatment guideline recommendation, providing that no more than one-third of the panel scored a scenario in the 1 to 3 range.
Dr FitzGerald then described some of the challenges the committee encountered. Some clinical questions have a lower evidence rating because there are little data to guide treatment. In other cases, it is difficult to tease out a single scenario among complex, interrelated topics. The guidelines have been criticized because (1) they do not address cost-effectiveness; (2) the evidence does not match the strength of the recommendation for some guidelines; and (3) some of the recommendations are controversial [Nuki G. Curr Opin Rheumatol. 2014]. Despite the controversies, however, Dr FitzGerald noted that the guideline-related quality measures have been added to the ACR's RISE Registry [NCT02230943] and that publication of the guidelines has brought more clinical attention to gout.
According to Kenneth G. Saag, MD, University of Alabama at Birmingham, Birmingham, Alabama, USA, guidelines should be advisory but not proscriptive and should combine the clinical state and circumstances, population values and preferences, and cost-effectiveness. He then highlighted the new method adopted by the ACR to develop guidelines, known as the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE).
GRADE is a transparent methodology that differentiates between conditional recommendations, typically based on low-quality evidence, and strong recommendations that are usually—but not always—based on at least moderate-quality evidence. The primary methodologic difference between RAND and GRADE is the additional development of PICO questions, which refers to Population (P), Intervention (I), Comparator (C), and Outcomes (O). Each PICO question defines the benefits and risks of a specific intervention, which helps guidelines teams capture evidence in a formal, evidence-based process.
A published appraisal of the 2012 gout guidelines compared them with 3 other sets of gout guidelines using an international scoring system [Nuki G. Curr Opin Rheumatol. 2014]. The ACR guidelines were given good scores for scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, editorial independence, and overall quality but not for applicability to practice.
Dr Saag then focused on 2 specific issues raised by the guidelines. The first is that febuxostat and allopurinol can both be considered first-line urate-lowering therapy (ULT). However, there are cost considerations associated with this guideline. In fact, published data suggest that feboxustat is not cost-effective compared with allopurinol as single-line therapy or as sequential therapy when febuxostat is prescribed first, but may be cost-effective when used sequentially after allopurinol (Table 2) [Jutkowitz E et al. Ann Intern Med. 2014].
Cost-Effectiveness of Gout Therapies
According to Dr Saag, there is also an issue regarding the appropriate starting dose of allopurinol. Because patients often receive doses of allopurinol inadequate to support clinical goals [Rashid N et al. Arthritis Rheum. 2011], and even patients who receive 300 mg of allopurinol may not achieve their target serum urate levels [Li-Yu J et al. J Rheumatol. 2001], there is concern that the current guideline (100 mg) may stall the process of uptitration. Despite the criticism of the 2012 guidelines, Dr Saag emphasized that ACR will continue to prioritize the development of treatment guidelines using the GRADE method and will keep seeking member input during the development process.
Robert Keenan, MD, Duke University School of Medicine, Durham, North Carolina, USA, highlighted the difficulties in implementing treatment guidelines. It has been estimated that only 50% of the guidelines developed across various therapeutic areas typically reach patients, in part because of contradictory recommendations from different associations that vary in quality [Lugtenberg M et al. BMC Fam Pract. 2011]. The treatment of gout appears to follow that same trajectory [Oderda GM et al. Postgrad Med. 2014; Harrold LR et al. Rheumatology. 2013].
There is no consensus as to why clinical guidelines are poorly implemented by health care providers. Barriers include a lack of familiarity with the guidelines, disagreement with the guidelines, resistance to change, limited time or resources, or clinical inertia. Likewise, there is no consensus how to best work with and encourage providers to implement guidelines.
Despite this lack of guidance, some strategies appear more likely to help providers implement clinical guidelines (Table 3). Dr Keenan also emphasized that the first step in implementing gout guidelines is changing the perception of gout among patients and providers, which will lead to higher expectations and better patient outcomes.
Strategies to Increase the Uptake of Gout Guidelines
- © 2014 MD Conference Express®