Summary
This article discusses a study was to evaluating the efficacy of ledipasvir/sofosbuvir fixed-dose combination in treatment-experienced patients with hepatitis C virus genotype 1 who failed to achieve SVR at week 12 with prior sofosbuvir-based therapy.
- Viral Infections Hepatology Clinical Trials
- Liver Conditions
- Viral Infections
- Hepatology
- Hepatology Clinical Trials
- Liver Conditions
Sofosbuvir plus pegylated interferon α (PEG-IFNα) and ribavirin for 12 weeks or sofosbuvir plus ribavirin for 24 weeks in PEG-IFNα-ineligible patients is approved for the treatment of patients with hepatitis C virus (HCV) genotype 1 (GT-1) infection. Patients who fail to achieve sustained viral response (SVR) with sofosbuvir-based therapies need effective treatment options. The objective of this study, presented by David L. Wyles, MD, University of California, San Diego, California, USA, was to evaluate the efficacy of ledipasvir/ sofosbuvir fixed-dose combination (FDC) in treatment-experienced patients with HCV GT-1 who failed to achieve SVR at week 12 (SVR12) with prior sofosbuvir-based therapy.
Ledipasvir is an HCV nonstructural 5A (NS5A) protein inhibitor, sofosbuvir is an HCV nonstructural 5B (NS5B) nucleotide polymerase inhibitor, and ledipasvir/sofosbuvir FDC is a once-daily, oral FDC (ledipasvir 90 mg/ sofosbuvir 400 mg) tablet.
A total of 51 patients who failed to achieve SVR12 in a previous sofosbuvir phase 2/phase 3 study were retreated with ledipasvir/sofosbuvir plus weight-based ribavirin. The primary end point was SVR12, defined as HCV RNA below the lower limit of quantification (LLOQ) at post-treatment week 12. The safety end points were adverse event (AE), discontinuation, and laboratory abnormality rates.
The mean patient age was 54 years and 61% of patients were men. The mean baseline HCV RNA log10 was 6.2 IU/ mL. Prior treatments included sofosbuvir plus PEG-IFNα/ ribavirin (n = 25), sofosbuvir with or without ribavirin (n = 21), and sofosbuvir-placebo plus PEG-IFNα/ribavirin (n = 5). Most patients were IL28B non-CC (92%) and GT-1a (59%).
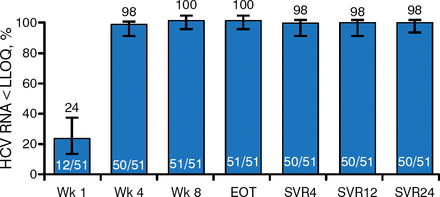
HCV RNA below the LLOQ was achieved by 24% of patients at week 1, 98% at week 4, and 100% of patients at week 8 and at the end of treatment (Figure 1). Fifty patients (98%) achieved SVR4, SVR12, and SVR24.
On-Treatment Viral Kinetics and Sustained Viral Response Rates
Error bars represent 95% confidence intervals.
HCV, hepatitis C virus; EOT, end of treatment; LLOQ, lower limit of quantification; SVR, sustained viral response.
Reproduced with permission from DL Wyles, MD.
The 1 patient who relapsed was incorrectly genotyped as GT-1 by line-probe assay at baseline (and in their prior treatment study). Viral sequencing subsequently determined that the patient was GT-3a at baseline and at the time of relapse. The patient had begun retreatment before the viral sequencing results were available.
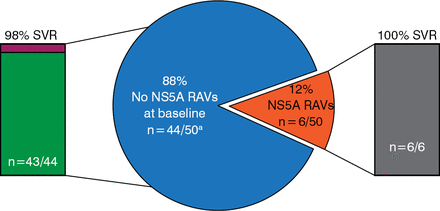
Resistance analysis showed that no patients had the sofosbuvir-associated variant S282T at baseline. Two patients had the NS5B treatment-emergent variant L159F at baseline and achieved SVR with retreatment. Six patients (12%) had NS5A resistance-associated variants at baseline and achieved SVR with retreatment (Figure 2).
Results of Baseline Resistance Analysis
NS5A, nonstructural 5A; RAV, resistance associated variant; SVR, sustained viral response.
aOne patient's baseline results were not available.
Reproduced with permission from DL Wyles, MD.
AEs were reported in 41 patients (80%), grade 3/grade 4 AEs were reported in 3 patients (6%), and serious AEs were reported in 2 patients (4%). One patient (2%) discontinued treatment due to AEs. Grade 3/grade 4 laboratory abnormalities occurred in 9 patients (18%). AEs occurring in ≥ 10% of patients were fatigue (26%), headache (22%), diarrhea (14%), rash (12%), insomnia (10%), and nausea (10%). Most AEs were mild or moderate in severity.
Among patients retreated with ledipasvir/sofosbuvir plus ribavirin for 12 weeks, 98% achieved SVR12. The 1 patient who relapsed had a GT-3a infection. Among patients with HCV GT-1 infection, 100% achieved SVR12. Ledipasvir/sofosbuvir plus ribavirin treatment for 12 weeks was safe and well tolerated. These data support this treatment regimen as an option for patients with HCV GT-1 infection who have failed to achieve SVR with prior sofosbuvir plus PEG-IFNα/ribavirin or sofosbuvir plus ribavirin regimens.
- © 2014 MD Conference Express®