Summary
This article presents initial data from A Placebo-Controlled, Double-Blind Study to Confirm the Reversal of Hepatorenal Syndrome Type 1 With Terlipressin [REVERSE; NCT01143246], the largest trial to date in type 1 hepatorenal syndrome using terlipressin.
- Hepatorenal Syndrome
- Hepatology Clinical Trials
- Liver Conditions
- Hepatorenal Syndrome
- Hepatology Clinical Trials
- Liver Conditions
- Hepatology
Thomas D. Boyer, MD, University of Arizona, Tucson, Arizona, USA, presented initial data from A Placebo-Controlled, Double-Blind Study to Confirm the Reversal of Hepatorenal Syndrome Type 1 With Terlipressin [REVERSE; NCT01143246], the largest trial to date in type 1 hepatorenal syndrome (HRS-1) using terlipressin. The results demonstrated that terlipressin plus albumin treatment improved renal function in patients with HRS-1 when compared with albumin alone.
According to Dr Boyer, HRS-1 involves the development of renal failure in patients with liver cirrhosis due to a sequence of events beginning with portal hypertension and development of a hyperdynamic circulation, leading to splanchnic and systemic vasodilation, and ultimately renal vasoconstriction.
Terlipressin is an arginine vasopressor (V1) receptor agonist that causes splanchnic and systemic vasoconstriction, redistributing blood to the arterial circulation, thereby improving renal perfusion. Albumin also augments this effect by helping to maintain the enhanced arterial blood volume.
The REVERSE trial was a randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy of terlipressin plus albumin for the treatment of HRS-1.
The primary end point was confirmed HRS reversal (CHRSR), defined as the number of patients with 2 serum creatinine values ≤ 1.5 mg/dL at least 48 hours apart while on treatment. Secondary end points included HRS reversal (1 serum creatinine value ≤ 1.5 mg/dL) and change in renal function as assessed by changes in serum creatinine.
Patients with HRS-1 were randomized 1:1 to treatment with terlipressin plus albumin (n = 93) or placebo (albumin alone; n = 95).
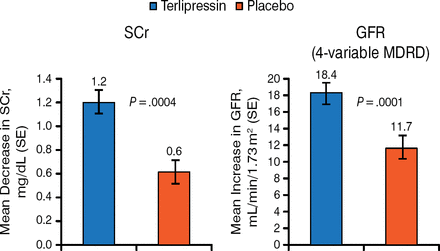
The primary end point of CHRSR was not met in the trial (19.6% vs 13.1%; P = .221). However, with respect to secondary end points, there was significant improvement in renal function in patients in the terlipressin group, with greater mean decreases in serum creatinine from baseline through the end of the study (1.2 vs 0.6 mg/dL; P = .0004). This corresponded to a significant increase in glomerular filtration rate with terlipressin (18.4 vs 11.7 mL/min/1.73 m2; P = .0001; Figure 1).
Improvement in Renal Function in the REVERSE Trial
SCr, serum creatinine; GFR, glomerular filtration rate; SE, standard error; MDRD, modification of diet in renal disease.
Reproduced with permission from TD Boyer, MD.
Improvements in serum creatinine were also found to correlate with patient survival at 90 days (P < .0001).
There was no significant difference in HRS reversal between the groups (23.7% vs 15.2%; P = .130).
The need for renal replacement therapy (RRT) was similar between the 2 groups. Among patients with CHRSR, none in the terlipressin group required RRT by 90 days, compared with about 25% of those who received placebo. The response to terlipressin may therefore be more durable, Dr Boyer suggested.
Overall survival was similar in both groups, with approximately half of the patients (56 vs 53) remaining alive at 90 days.
The incidence of adverse events (AEs) was consistent with those reported in previous trials using terlipressin. Additionally, as expected with the use of a vasoactive drug, significantly more patients in the terlipressin group required treatment discontinuation because of AEs (20.4% vs 6.3%; P = .008).
Dr Boyer concluded that terlipressin treatment has a favorable benefit-risk profile in HRS-1, and, compared with placebo, is associated with significant improvement in renal function that also correlates with improved survival.
- © 2014 MD Conference Express®