Summary
This article presents results from the Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Subjects With Nonvalvular Atrial Fibrillation Scheduled for Cardioversion trial [X-VeRT; Cappato R et al. Eur Heart J. 2014]. The data showed that oral rivaroxaban was as effective and safe as a vitamin K antagonist when administered for only about a week prior to elective cardioversion for atrial fibrillation.
- Cardiology
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Arrhythmias
- Cardiology
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Arrhythmias
Riccardo Cappato, MD, Policlinico San Donato, San Donato Milanese, Italy, presented results from the Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Subjects With Nonvalvular Atrial Fibrillation Scheduled for Cardioversion trial [X-VeRT; Cappato R et al. Eur Heart J. 2014]. The data showed that oral rivaroxaban was as effective and safe as a vitamin K antagonist (VKA) when administered for only about a week prior to elective cardioversion for atrial fibrillation (AF).
According to Prof Cappato, although cardioversion is commonly performed worldwide to restore normal rhythm in patients with AF [Hernández-Madrid A et al. Europace 2013], without appropriate anticoagulation therapy, the periprocedural risk of thromboembolism for this procedure is 5% to 7% [Stellbrink C et al. Circulation 2004], compared with 1% for patients who receive a VKA [Gallagher Mm et al. J Am Coll Cardiol. 2002], the current standard of care pre- and post-cardioversion [Camm AJ et al. Eur Heart J. 2013].
X-VeRT is the first prospective randomized study designed to compare the efficacy and safety of a novel oral anticoagulant (NOAC) with dose-adjusted VKAs in patients with AF undergoing elective cardioversion. This open-label, parallel-group, active-controlled phase 3b trial enrolled 1504 subjects with hemodynamically stable nonvalvular AF who were scheduled for cardioversion from 141 centers throughout 16 countries.
Patients were randomized 2:1 to rivaroxaban 20 mg once daily (15 mg if creatinine clearance was 30 to 49 mL/min) or international normalized ratio (INR)-adjusted VKA therapy, including warfarin. Local study investigators decided whether patients underwent an early (target period of 1 to 5 d post randomization) or delayed (3 to 8 weeks) cardioversion strategy.
The primary efficacy outcome was the composite of stroke and transient ischemic attack (TIA), non—central nervous system (CNS) systemic embolism, myocardial infarction, and cardiovascular death. The primary safety outcome was major bleeding, according to International Society on Thrombosis and Haemostasis recommendations.
The primary efficacy outcome occurred in 0.51% of the rivaroxaban group and 1.02% of the VKA group (RR, 0.50; 95% CI, 0.15 to 1.73). In patients who underwent early cardioversion, the primary composite outcome occurred in 0.71% of the rivaroxaban group vs 1.08% of the VKA group, and in 0.24% vs 0.93% of those who underwent delayed cardioversion.
The incidence of major bleeding was similar in patients treated with rivaroxaban and VKAs (0.6% vs 0.8%; RR, 0.76; 95% CI, 0.21 to 2.67).
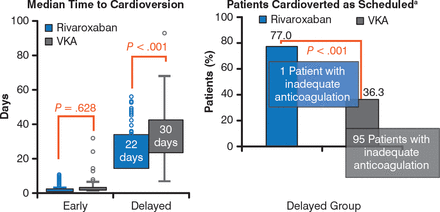
In patients selected for early cardioversion, time to cardioversion was similar in both treatment groups (P = .628), but in those selected for delayed cardioversion, it was significantly shorter in patients who received r ivaroxaban compared with VKAs (P < .001; Figure 1).
Effects of Rivaroxaban and Vitamin K Antagonists on Time to Cardioversion
VKA, vitamin K antagonist; INR, international normalized ratio. a Reason for not performing cardioversion as first scheduled from 21–25 days primarily due to inadequate anticoagulation (indicated by drug compliance <80% for rivaroxaban or weekly INRs outside the range of 2.0–3.0 for 3 consecutive weeks before cardioversion for VKA).
Reproduced with permission from R Cappato, MD.
Prof Cappato emphasized that differences in the primary efficacy and safety outcomes between the groups were not significant because this study was not powered to determine statistical significance. He concluded, however, that the results thus far suggest reassuring efficacy and safety profiles for rivaroxaban, and imply that this NOAC may represent a promising alternative to VKAs, allowing prompt, elective cardioversion in patients with A F.
- © 2014 MD Conference Express®