Summary
Acute spinal cord injury (SCI) is a devastating injury that can be the result of varying traumatic mechanisms. While early surgical decompression and different pharmacological agents have been advocated over the years, it is still unclear how effective these treatments are in improving neurological outcomes. This article discusses the ongoing Riluzole in Spinal Cord Injury Study [RISCIS; NCT01597518], a phase 3, multicenter, double-blind, randomized controlled trial to evaluate the efficacy and safety of off-label use of riluzole to treat patients with acute SCI.
- Trauma
- Spine Conditions Orthopaedics Clinical Trials
- Trauma
- Spine Conditions
- Orthopaedics
- Orthopaedics Clinical Trials
Acute spinal cord injury (SCI) is a devastating injury that can be the result of varying traumatic mechanisms. While early surgical decompression and different pharmacological agents have been advocated over the years, it is still unclear how effective these treatments are in improving neurological outcomes. Thus, there is a continued need for further work in this area.
Michael G. Fehlings, MD, University of Toronto, Toronto, Ontario, Canada, described the rationale and design of the ongoing Riluzole in Spinal Cord Injury Study [RISCIS; NCT01597518], a phase 3, multicenter, double-blind, randomized controlled trial to evaluate the efficacy and safety of off-label use of riluzole to treat patients with acute SCI. The trial was initiated based on the preliminary data from the phase 1/2a trial [NCT00876889] showing the safety and efficacy of riluzole in this setting. This established the feasibility of a multicenter trial to evaluate riluzole for traumatic SCI.
The phase 1/2a trial compared 36 patients with traumatic SCI treated with riluzole and 36 control patients in a registry cohort but did not find a significant difference in complications between the groups (Table 1).
Complications in the Riluzole Registry Groups
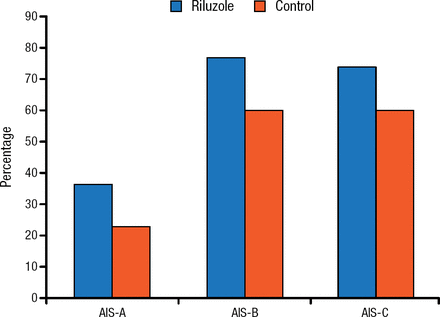
In addition, the study found no between-group difference in the percent conversion of the American Spinal Injury Association Impairment Scale grade (Figure 1).
Percent Conversion of AIS Grade
AIS, American Spinal Injury Association Impairment Scale. Reproduced with permission from MG Fehlings, MD.
Based on these preliminary results, the ongoing phase 3 trial [NCT01597518] will evaluate the safety and efficacy of riluzole in the treatment of traumatic SCI. The primary outcome of the study is the change in the International Standards for Neurological Classification of SCI (ISNCSCI) total motor score from baseline to 180 days. Secondary outcomes are measures of ISNCSCI grade, ISNCSCI sensory scores, spinal cord independence measure, Short Form-36 Health Survey version 2.0, EuroQol health outcomes measure, pain numeric rating scale, and graded and redefined assessment of strength, sensibility, and prehension.
Enrollment into the phase 3 trial began in January 2014. Patients with acute traumatic SCI (n = 351) from 35 international sites will be randomized in a 1:1 fashion to riluzole 100 mg BID for 24 hours, followed by riluzole 50 mg BID for 13 days after the injury or to the same dosing regimen of placebo.
Patients included in the study must be able to receive the study drug within 12 hours of injury, have an ISNCSCI impairment scale grade of A, B, or C, and have a neurological level of injury C4 to C8 based on the first ISNCSCI assessment after arrival to the hospital.
Patients excluded from the study are those with an injury arising from a penetrating mechanism and those with significant concomitant head injury.
According to Dr Fehlings, the study will use an adaptive sequential design to allow sample size changes during the interim analysis. To date, the study has enrolled 11 patients. Dr Fehlings hopes to come up with a neuroprotective strategy that could influence clinical practice in treatment of SCI.
- © 2015 MD Conference Express®