Summary
This article iscussed advances in radio-frequency ablation for osteoid osteoma and other benign bone tumors. Other topics include cryoablation and microwave ablation for the treatment of bone metastases, and magnetic resonance–guided focused ultrasound for palliative treatment of painful bone metastases.
- tumors
- interventional radiology
Mark Richard Robbin, MD, University Hospitals, Cleveland, Ohio, USA, discussed advances in radiofrequency ablation (RFA) for osteoid osteoma and other benign bone tumors. RFA involves the insertion of an electrode into the target tissue under medical imaging guidance. The electrode is then heated with a radiofrequency electric current to destroy the target tissue.
Dr Robbin performs RFA under general anesthesia and uses preprocedural thin-slice computed tomography (CT) for access planning. He administers 2 sequential 6-minute ablations at 90°C. A retrospective study of this technique demonstrated a 100% short-term success rate and a 0% recurrence rate at 5.2 (1.0 to 12.7) years, with 1 delayed stress injury after early return to sports [Abboud S et al. SIR 2014 (abstr 239)].
A study of CT-guided RFA for nonspinal osteoid osteomas reported that a dual ablation cycle decreased tissue impedance, had greater potential for killing peripheral residual tumor foci, allowed electrode repositioning, and resulted in greater success rates (P < .001; Table 1) [Rimondi E et al. Eur Radiol. 2012]. Gradually increasing the temperature and prolonging ablation times also improved outcomes.
Improved Outcomes With Dual-Cycle Radiofrequency Ablation for Osteoid Osteomas
Heat Generation in Tissue With Radiofrequency and Microwave Ablation
According to Dr Robbin, a 6-minute ablation may not be sufficient for treating recurrences and larger lesions. Margins can be difficult to define in postsurgical recurrences. The volume and longitudinal extent of the lesion must be calculated to define the complete ablation zone.
Selected cases of chondroblastoma can be treated with RFA. In one study, 12 of 14 patients were pain free after long-term follow-up [Rybak LD et al. Radiology. 2009]. Giant cell tumors and aneurysmal bone cysts may also be treated with RFA.
Damian E. Dupuy, MD, Brown University, Providence, Rhode Island, USA, discussed cryoablation and microwave ablation (MWA) for the treatment of bone metastases. The goals of ablation are to achieve local control and pain palliation. Ablation can be used in combination with radiation therapy and vertebroplasty.
The cryoablation technique employs a rapid freeze and slow-thaw cycle, resulting in cell shrinkage and rupture and small blood vessel clotting. The cytotoxic threshold is −40°C. Cryoablation systems are argon based. The ice ball is visible with CT, ultrasound (US), and magnetic resonance imaging (MRI). Cryoablation is relatively painless during treatment. Larger ablation volumes can be achieved than with RFA, without disruption of collagenous architecture. Treated tissue is not denatured, allowing quicker absorption of dead tissue.
The advantages of cryoablation include the following:
-
Ice ball visualization
-
Lethal zone 3 to 5 mm within margin
-
Treatment close to critical structures
-
Relatively painless
-
No need for general anesthesia
A multicenter trial of percutaneous image-guided cryoablation of painful bone metastases reported that treatment with cryoablation significantly decreased the mean score for worst pain (P < .0001) and there was 1 postprocedural osteomyelitis [Callstrom MR et al. Cancer. 2013].
Thermal myositis is a potential adverse effect, especially in larger ablation zones, but usually it is asymptomatic; however, pain medication may be needed in some cases [Bing F et al. AJR Am J Roentgenol. 2014].
MWA is performed with high-frequency electromagnetic radiation at 1 or 2.45 GHz. The oscillation of polar molecules produces frictional heating without an electrical current. Table 2 compares the heat generation of MWA with RFA.
MWA has the following advantages over RFA: The use of multiple applicators increases treatment flexibility, large tissue volumes can be ablated in shorter time periods [Laeseke PF et al. J Vasc Interv Radiol. 2009], the heat sink effect is much smaller [Dodd GD et al. Radiology. 2013], MWA may be more effective [Brace CL et al. Radiology. 2009], and MWA appears to be less painful. In a study of MWA for painful bone metastases, the Brief Pain Inventory score declined by 92% during 3 months of follow-up [Pusceddu C et al. J Vasc Interv Radiol. 2013].
Dramatic and persistent pain reduction with MWA also has been demonstrated in patients with painful refractory spinal metastases [Kastler A et al. J Vasc Interv Radiol. 2014].
The evidence demonstrates that bone cryoablation and MWA are safe and appear promising for palliation of metastatic bone disease. Margin control and low pain are key advantages of cryoablation. An important advantage of MWA is the fast treatment time, even in large tumors. Further clinical studies of both procedures are warranted.
David Gianfelice, MD, University Health Network, Toronto, Ontario, Canada, addressed magnetic resonance–guided focused ultrasound (MRgFUS) for palliative treatment of painful bone metastases. External beam radiation is the gold standard for treatment of bone metastases, but at least 30% of patients do not get adequate pain relief. MRgFUS is performed with an US transducer located in a standard MR table without needle or probe placement. High-energy US waves increase the temperature in target tissues, which become necrotic above 60°C. Heating bone above 60°C effectively destroys the periosteal innervation for pain palliation. Because FUS energy absorption by bone is approximately 50 times greater than that of soft tissue, bone can be treated with a low-energy, wide-beam approach for a short treatment time.
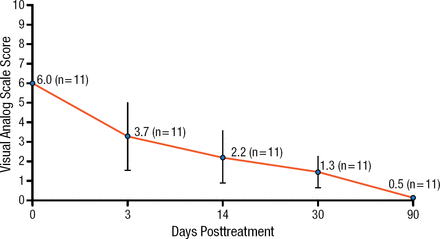
A pilot study of MRgFUS in 11 patients with bone metastases reported a progressive reduction in pain and pain medication usage in all patients during the 3-month follow-up, with a 92% decrease in pain scores (P < .01; Figure 1) [Gianfelice D et al. Radiology. 2008]. No adverse events were reported.
Pain Palliation with Magnetic Resonance–Guided Focused Ultrasound Treatment of Bone Metastases
Adapted from Gianfelice D et al. Palliative treatment of painful bone metastases with MR imaging–guided focused ultrasound. Radiology. 2008;249:355–363. With permission from the Radiological Society of North America.
The pivotal phase 3 trial of MRgFUS demonstrated that MRgFUS is safe and effective for pain palliation in patients with metastatic bone lesions who were not suitable candidates for radiation therapy [Hurwitz MD et al. J Natl Cancer Inst 2014]. The response rate at 90 days was 64.3% in the MRgFUS arm vs 20.0% in the placebo arm. Quality-of-life improvement was clinically significant. The most common treatment-related adverse event was sonication pain (32.1%).
MRgFUS treatment of bone metastases does not require image-guided interventional skills. It is effective for short-term pain relief and is a viable palliative treatment. MRgFUS is an outpatient procedure with few or no adverse events and is repeatable with little morbidity. Future treatment protocols aim to achieve complete ablation of the medullary component of osteolytic metastases to achieve local tumor control and pain palliation.
- © 2015 MD Conference Express®