Summary
Different types of cancer have distinct DNA methylation profiles, and analyses of tumor samples have found that multiple genetic and epigenetic changes are associated with breast cancer. As some epigenetic changes may be reversible, administering epigenetic agents with other therapies may be potent combinations in the treatment of breast cancer.
- DNA methylation
- polycomb-binding adult stem cells
- clinical trials
- therapy
- tamoxifen
- vorinostat
- exemestane 5-azacitidine
- entinostat
- ENCORE 301
- E2112 trial
- NCT02115282
Epigenetics is a broad term used to describe structural changes in DNA that activate or inactivate specific genes. DNA methylation is one type of epigenetic change in which a methyl group is added to DNA that prevents gene expression. Peter W. Laird, PhD, Van Andel Research Institute, Grand Rapids, Michigan, USA, gave an overview of epigenetics in breast cancer (BC), focusing primarily on DNA methylation.
Focal hypermethylation is seen in cancer cells, which can lead to transcriptional silencing. The Cancer Genome Atlas (http://cancergenome.nih.gov/) contains > 25 major types of cancer, with > 10 000 primary cancer specimens. Analyses of mutation, expression, copy number, mRNA, reverse-phase protein assays, and DNA methylation have been performed on these samples.
Dr Laird’s laboratory team analyzed DNA hypermethylation patterns in 892 normal tissues and about 5000 primary tumor tissues. The results indicated that different cancers have very distinct methylation profiles. Further investigation revealed that a single DNA-binding protein (EZH2) appeared to drive abnormal DNA methylation. This protein is part of the polycomb repressive complex and helps to control the activity of master regulators of differentiation and development. Dr Laird noted that there are about 2000 genes in the human genome that control age-specific differentiation and development. Many of these genes are kept in check by polycomb repressive complexes early in development.
One possible model explaining this finding concerns the profile of polycomb-binding adult stem cells [Widschwendter M et al. Nat Genet. 2007]. Master regulators of differentiation and development are not actively transcribed because they are transiently repressed by the polycomb complexes. During normal differentiation, the polycomb repression is removed, tissue-specific gene expression is turned on, and the cell differentiates. As a person ages, some of these polycomb-occupied sites acquire an abnormal mark of DNA methylation, and the polycomb is lost. According to Dr Laird, there is a switch from polycomb to DNA methylation, which is a much more permanent silencing of transcription. Because the master regulator can no longer be activated, the cell cannot differentiate properly and is vulnerable to malignant transformation.
Multiple genetic and epigenetic changes contribute to BC. Inactivated tumor suppressor genes and other genes that are silenced via methylation help establish and maintain tumors. However, some epigenetic changes may be reversible, and these pathways are therefore attractive clinical targets. Agents that inhibit DNA methyltransferases or histone deactylase (HDAC) help re-establish gene expression and function, leading to greater sensitivity to antitumor therapies [Connolly R, Stearns V. J Mammary Gland Biol Neoplasia. 2012]. Vered Stearns, MD, Johns Hopkins University, Baltimore, Maryland, USA, provided an overview of clinical investigations of epigenetics in BC. Promising combinations with HDAC inhibition in BC epigenetics include endocrine therapy and demethylating agents.
Combining endocrine therapy with HDAC inhibition may be a promising BC therapy. In a phase 2 study [Munster PN et al. Br J Cancer. 2011] of 43 patients with estrogen receptor (ER)-positive metastatic BC, the combination of tamoxifen and the HDAC inhibitor vorinostat led to an objective response rate (ORR) of 19% and a clinical benefit rate of 40%. In the randomized phase 2 ENCORE 301 study [Yardley DA et al. J Clin Oncol. 2013], patients with ER-positive advanced BC were randomized to treatment with exemestane plus entinostat (n = 64) or exemestane plus placebo (n = 66). The median progression-free survival (PFS) was 4.3 months in the combination group vs 2.3 months in the exemestane-only group (HR, 0.73; 95% CI, 0.50 to 1.07; 1-sided P = .055; 2-sided P = .11). OS was significantly better in the combination group (median 28.1 months) vs exemestane only (median 19.8 months; HR, 0.59; 95% CI, 0.36 to 0.97; P = .036). Interestingly, the results were consistent with results in other epigenetic trials, such as lung cancer trials, demonstrating that it may be that an epigenetic therapy could sensitize the tumor cells to other treatments. In addition, acetylation was found to be a possible biomarker of response, as women with an acetylation signature were more likely to benefit from the exemestane/entinostat combination.
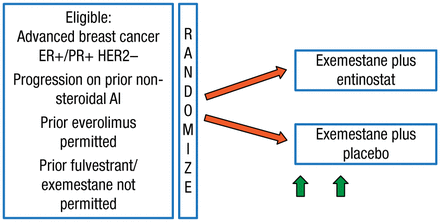
These findings led to the design of the phase 3 E2112 trial [NCT02115282], which is currently enrolling patients. The study schematic is illustrated in Figure 1.
Schematic for Study E2112
AI, aromatase inhibitor; ER+, estrogen receptor–positive; HER2–, human epidermal growth factor receptor 2–negative; PR+, progesterone receptor–positive.
Reproduced with permission from V Stearns, MD.
This E2112 study will randomize 600 postmenopausal patients with advanced hormone receptor-positive/human epidermal growth factor receptor 2-negative BC. Patients will receive exemestane 25 mg daily plus entinostat 5 mg weekly or exemestane plus placebo. The hypothesis is that the addition of the HDAC inhibitor will improve PFS and OS in these patients. Secondary end points include safety and tolerability, ORR, time to treatment deterioration, overall health-related quality of life, protocol adherence, and whether the response to exemestane plus entinostat is correlated with changes in peripheral blood mononuclear cell acetylation status.
An open-label phase 2 study [Connolly RM et al. ASCO 2014 (abstr 569)] of the demethylation agent 5-azacitidine (5-AZC) and the HDAC inhibitor entinostat in patients with advanced BC has completed enrollment. Patients received 28-day treatment cycles of 5-AZC 40 mg/m2 subcutaneously on days 1 to 5 and 8 to 10 combined with entinostat 7 mg on days 3 and 10. The study was divided into 2 cohorts: triple-negative BC (TNBC) and hormone-resistant BC. Patients with progressive disease could enroll in an optional continuation phase and receive 5-AZC plus entinostat plus hormonal therapy at the physician’s discretion.
Thirteen TNBC patients and 27 hormone-resistant patients participated in the study. No responses were seen in the first stage in the TNBC cohort and it was closed to accrual. The median PFS was 1.4 months (95% CI, 0.9 to 1.8) and the median overall survival (OS) was 6.6 months (95% CI, 2.0 to 10.3) [Connolly RM et al. AACR 2013 (abstr 4666)]. In the hormone-resistant cohort, the median PFS was 1.8 months (95% CI, 1.7 to 1.9), with a response rate of 4% [Connolly RM et al. ASCO 2014 (abstr 569)]. One patient had a partial response after 2 and 4 cycles but subsequently progressed at cycle 6, and 1 patient had a partial response during the optional continuation phase. Correlative analyses of serial biopsy specimens are ongoing. Dr Sterns noted that although the primary end point was not met, the findings show that there may be a role for further evaluation in hormone-resistant BC.
- © 2014 SAGE Publications