Summary
Results from the MR CLEAN clinical trial demonstrated that intra-arterial treatment within 6 hours of stroke onset in patients with acute ischemic stroke due to intracranial anterior occlusion was safe and effective at significantly reducing disability at 90 days, infarct volume at 7 days, and residual occlusion at 24 hours poststroke.
- acute ischemic stroke
- endovascular
- disability
- infarct volume
- intra-arterial
- MR CLEAN
- residual occlusion
Yvo B. Roos, MD, PhD, University of Amsterdam, Amsterdam, the Netherlands, presented the results from the MR CLEAN trial [Berkhemer OA et al. N Engl J Med. 2015], which demonstrated, for the first time, the safety and efficacy of intra-arterial treatment within 6 hours of stroke onset in patients with acute ischemic stroke caused by intracranial anterior circulation occlusion.
MR CLEAN was a multicenter, randomized, prospective, open-label, phase 3 trial with blinded assessment conducted in the Netherlands. Eligible patients were aged ≥ 18 years with a National Institutes of Health Stroke Scale (NIHSS) score of ≥ 2, who had an acute ischemic stroke due to intracranial anterior circulation occlusion and received treatment within 6 hours from stroke onset.
Intra-arterial intervention consisted of arterial catheterization with a microcatheter to the level of the intracranial arterial occlusion, followed by delivery of a thrombolytic agent, mechanical treatment, or both (the method was left to the discretion of the treating physician). The primary outcome was the score on the modified Rankin Scale (mRS) at 90 days. Secondary outcomes included neuroimaging of arterial recanalization at 24 hours and the final infarct volume at 7 days.
The study enrolled a total of 500 patients (233 in the intervention group, 267 in the control group). The main clinical characteristics, including the mean age and NIHSS score, at baseline were similar between the groups. Intra-arterial therapy was performed in 196 of the 233 patients within the intervention group. Retrievable stents were used in 190 of these patients (97%), other devices were used in 5 patients (2.6%), and only 1 patient (0.4%) received thrombolytic treatment alone.
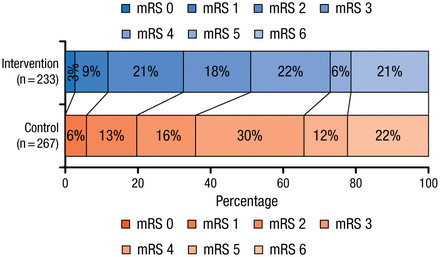
The primary outcome analysis revealed that there was a shift in the distribution of the mRS scores at 90 days in favor of the intervention (OR, 1.67; 95% CI, 1.21 to 2.30), which was consistent for all mRS categories except death (Figure 1). The distribution of mRS scores of 6 (death) were similar in both groups.
mRS Score Distribution at 90 Days
mRS, modified Rankin Scale.
From N Engl J Med, Berkhemer OA et al, Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke, 2015;372:11-20. Copyright © Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Computed tomographic angiography at 24 hours demonstrated that residual occlusion at the target site could not be detected in 75.4% of patients in the intervention group vs 32.9% in the control group (OR, 6.9; 95% CI, 4.3 to 10.9). The between-group difference in the final infarct volume at 7 days also favored the intervention group (19 mL; 95% CI, 4 to 34). The NIHSS score at 7 days was, on average, 2.9 points lower in the intervention group than in the control group (95% CI, 1.5 to 4.3). Prespecified subgroup analyses demonstrated that the treatment effect remained consistent regardless of age, NIHSS score, time from onset to randomization, and other criteria.
There was no significant difference between the intervention and the control group in the occurrence of serious adverse events, such as parenchymal hemorrhage type 2, pneumonia, and hemicraniectomy. The only notable difference was a higher incidence of new ischemic stroke in different vascular territory in the intervention group (5.6%) vs the control group (0.4%); however, according to Prof Roos, that is an expected adverse effect of mechanical intervention.
- © 2015 SAGE Publications