Summary
Understanding the underlying pathology of Parkinson's disease (PD) is critical in identifying the disease early and developing novel treatments. This article discusses the role of a-synuclein in PD, the extranigral pathology and preclinical detection of PD, as well as how the updated understanding of PD pathology explains its clinical phenotype
- Extrapyramidal & Movement Disorders
- Dementias
- Extrapyramidal & Movement Disorders
- Neurology
- Dementias
Understanding the underlying pathology of Parkinson's disease (PD) is critical in identifying the disease early and developing novel treatments. John Trojanowski, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA, described the role of α-synuclein in PD. The misfolding of proteins, such as α-synuclein, can impart toxic properties. For example, intraneuronal Lewy bodies, which are composed of misfolded α-synuclein amyloid fibers, are present in the brains of patients with PD. In addition, the development of cognitive impairment is a feature of PD that coincides with the progression of Lewy bodies [Irwin DJ et al. Ann Neurol 2012], and indeed, many patients with PD will develop dementia. In addition to Lewy body pathology, Alzheimer's pathology is observed in patients with PD.
Researchers of a pilot study compared cerebrospinal fluid from patients with PD to spinal fluid from healthy controls to identify biomarkers for PD. Measurements of α-synuclein, β-amyloid1–42, total tau, and tau phosphorylated at threonine 181 were significantly lower in patients with PD compared with healthy controls (p<0.05) [Kang JH et al. JAMA Neurol 2013].
With regard to animal studies, an α-synuclein transgenic mouse model showed normal spontaneous motor activity and cued fear conditioning but impaired contextual fear conditioning, indicating an effect on the hippocampus [Lim Y et al. J Neurosci 2011]. Additionally, a time-dependent increase in a rapidly progressive neurodegenerative α-synucleinopathy has been observed in mice that received an intracerebral inoculation of pathologic α-synuclein [Luk KC et al. J Exp Med 2012]. The pathology seen in these mice was similar to that observed in PD. In a third study, human corticobasal degeneration brain lysates of pathologic tau injected into healthy 3-month-old mice resulted in tau aggregate formation in hippocampal neurons and white matter [Iba M et al. J Neurosci 2013], which is similar to the pathology observed in Alzheimer's disease. These and other studies suggest a new understanding of PD, in that its transmission occurs from cell to cell, which could explain its progression in humans.
Virginia Lee, PhD, MBA, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA, discussed how the updated understanding of PD pathology explains its clinical phenotype. Fibrillar α-synuclein acts as intracellular seeds that eventually form Lewy body–like α-synuclein aggregates in the soma of the affected neuron. When preformed α-synuclein fibrils (PFFs) are administered to wild-type mice, they promote endogenous α-synuclein to form pathologic aggregates. As discussed above, intrastriatal injection of mouse PFFs into mice results in the formation of Lewy bodies [Luk KC et al. Science 2012]. Additionally, a seeded pathology that resembles Lewy pathology can cause loss of synapses and synaptic proteins and impair normal cellular function in cultured neurons from wild-type mice [Volpicelli-Daley LA et al. Neuron 2011].
Different strains of PFFs have been generated from recombinant α-synuclein protein; one strain is able to recruit tau and the other cannot, in vitro and in mice [Guo JL et al. Cell 2013]. Biochemical and molecular analyses indicated that there are conformational differences between the α-synuclein strains and that more N- and C-termini are exposed in strain B. Strain-specific monoclonal antibodies support the theory of a conformation difference between the 2 α-synuclein strains by enzyme-linked immunosorbent assay [Guo JL et al. Cell 2013].
Dr. Lee suggested that if misfolded α-synuclein can be transmitted to healthy cells and cause disease, perhaps the uptake of misfolded α-synuclein can be blocked via immunotherapy. In vitro studies revealed that anti-α-synuclein monoclonal antibodies resulted in a decrease of PFF-induced insoluble pathologic α-synuclein aggregates in primary neurons, as well as synaptic loss and neuron death [Tran HT et al. Cell Rep 2014]. The antibodies also blocked the uptake of α-synuclein PFFs and prevented cell-to-cell transmission of misfolded α-synuclein. Also, the anti-α-synuclein antibody Syn303 has been observed to prevent the spread of Lewy pathology and decreased motor deficits and aggregate formation in mice that received an intrastriatal PFF injection.
Charles H. Adler, MD, PhD, Mayo Clinic College of Medicine, Scottsdale, Arizona, USA, discussed extranigral pathology and preclinical detection of PD. There are 4 proposed stages of Parkinson's at-risk syndrome: prephysiologic, preclinical, premotor, and prediagnostic [Siderowf A, Stern MD. Ann Neurol 2008]. The prephysiologic stage is characterized by patients who have a genetic risk to develop PD, but no symptoms have yet developed; however, mutations or triplication of the synuclein gene and mutations of the leucine-rich repeat kinase 2 gene. In the preclinical phase, there are still no clinical signs of PD, but patients may be positive for biomarkers. While still being investigated, examples may include imaging studies such as transcranial ultrasound, 123I-MIBG scintigraphy, radioligand tracers of presynaptic dopamine function, and magnetic resonance imaging–diffusion tensor imaging.
Patients begin to develop nonmotor signs or symptoms of PD in the premotor phase. Researchers of a longitudinal clinicopathologic study [AZSAND; Adler C et al. Neurology 2014] evaluated annual movement, neuropsychology, sleep, autonomic function, as well as performance on the University of Pennsylvania Smell Test (UPSIT) every third year, following subjects until death and autopsy.
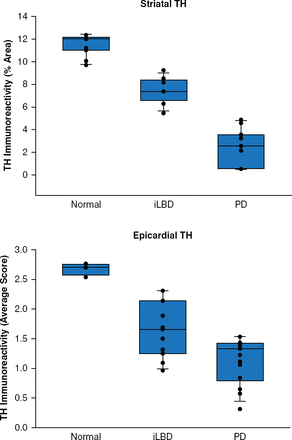
Focusing on preclinical detection of PD, Dr. Adler next reviewed incidental Lewy body disease (iLBD), which is characterized by the presence of Lewy bodies without parkinsonism or dementia. The prevalence of iLBD increases with age, as ≤12% of individuals aged >60 years and ≤30% of autopsied elderly patients have iLBD. Dr. Adler suggested that these patients with iLBD may actually have preclinical PD or dementia with Lewy bodies (DLB). Patients with iLBD have a lower level of tyrosine hydroxylase (TH) compared with healthy controls (p=0.01) [Beach TG et al. Acta Neuropathol 2008]. In addition, striatal and epicardial levels of TH are between those of healthy controls and patients with PD (Figure 1) [Dickson DW et al. Acta Neurophathol 2008].
Tyrosine Hydroxylase Levels in the Striatum and Epicardium in iLBD
iLBD=incidental Lewy body disease; PD=Parkinson's disease; TH=tyrosine hydroxylase.
Reproduced from Dickson DW et al. Evidence that incidental Lewy body disease is presymptomatic Parkinson's disease. Acta Neuropathol 2008;115:437–444. With permission from Springer Verlag.
In a case-control study, patients with iLBD also had significantly lower UPSIT and Trails B scores compared with patients without iLBD (p≤0.001) [Adler CH et al. Mov Disord 2010]. Last, patients with iLBD score lower in the 12-odor Cross-Cultural Smell Identification Test compared with controls [Ross GW et al. Mov Disord 2006].
Hyposmia is well documented in patients with PD but is not specific for it. In the Parkinson At-Risk Study, ∼5000 patients without PD performed UPSIT, and 13.4% were found to be hyposmic [Siderowf A et al. Mov Disord 2012]. In addition, constipation, depression, anxiety, and rapid eye movement (REM) sleep behavior disorder (RBD) were associated with hyposmia. Data of the conversion rate to PD are expected in the future. RBD is present in the majority of patients with PD, and the majority of patients with idiopathic RBD develop PD or DLB. In addition, pathologic data suggest that RBD is a synucleinopathy. Reduced olfactory function and color vision in patients with RDB is associated with neurodegeneration [Iranzo A et al. Parkinsonism Relat Disord 2013; Postuma RB et al. Ann Neurol 2011]. In addition, in a longitudinal study, 20 of 78 patients with RBD developed parkinsonism with motor findings preceding the diagnosis of parkinsonism by ≤5 years [Postuma RB et al. Brain 2012].
Although it has low specificity, constipation is a highly sensitive indicator of PD [Postuma RB et al. Mov Disord 2012], as men with <1 bowel movement a day had a ≥4-fold risk of developing PD compared with men who had ≥2 bowel movements a day [Abbott RD et al. Neurology 2001]. In addition, 24% of men with iLBD had <1 bowel movement a day, compared with 6.5% of men with >1 bowel movement a day [Abbott RD et al. Mov Disord 2007]. Lewy bodies have been found in the myenteric plexus of the esophagus and colon, and accumulation of α-synuclein in the bowel of patients with PD and preclinical PD has been reported [Beach et al. Acta Neuropathol 2010; Hilton D et al. Acta Neuropathol 2014; Shannon KM et al. Mov Disord 2012].
In conclusion, PD is a progressive disorder that begins before the onset of symptoms. As reviewed, misfolded α-synuclein, a hallmark of PD, can be transmitted from cell to cell and cause aggregation of α-synuclein and tau protein in healthy neurons. Mutations in the synuclein, leucine-rich repeat kinase 2, Parkinson protein 2, PTEN-induced putative kinase 1, and glucosidase beta acid genes, among others, can result in the development of PD. Ideally, as preclinical pathology and symptoms are clarified, this can open up avenues for optimizing early diagnosis and treatment.
- © 2014 MD Conference Express®