Summary
In patients with relapsing-remitting multiple sclerosis (RRMS), bimonthly injection with low-dose aB-crystallin (HspB5) resulted in a decrease in gadolinium-enhancing (Gd+) lesions and clinical relapses as compared with the placebo. This article presents data from a randomized study [2011-004475-36; van Noort JM et al. ACTRIMS/ECTRIMS 2014 (poster P082)] that evaluated HspB5 for the treatment of RRMS.
- Demyelinating Diseases
- Neurology Clinical Trials
- Demyelinating Diseases
- Neurology Clinical Trials
- Neurology
In patients with relapsing-remitting multiple sclerosis (RRMS), bimonthly injection with low-dose αB-crystallin (HspB5) resulted in a decrease in gadolinium-enhancing (Gd+) lesions and clinical relapses as compared with the placebo. Hans van Noort, PhD, Delta Crystallon, Leiden, the Netherlands, presented data from a randomized study [2011-00447536; van Noort JM et al. ACTRIMS/ECTRIMS 2014 (poster P082)] that evaluated HspB5 for the treatment of RRMS.
The oligodendrocytes of patients with MS have high levels of HspB5, a glial stress protein that initiates local anti-inflammatory, neuroprotective, and tolerogenic innate responses [van Noort JM et al. J Neuropathol Exp Neurol. 2010; Ousman SS et al. Nature. 2007]. However, interferon-gamma-secreting Th1 memory cells that target HspB5 exist [van Noort JM et al, Nature. 1995; Ousman SS et al. Nature. 2007; Bajramović JJ et al. J Immunol. 2000], which results in interferon-gamma-induced tissue damage [Bsibsi M et al. Acta Neuropathol. 2014]. In a previous Phase 1 study, administration of a single injection of HspB5 to healthy subjects led to an antigen-specific decrease in T-cell responses. The purpose of this study was to further evaluate low doses of HspB5 in patients with RRMS.
In this double-blind phase 2a trial, 32 patients with RRMS were randomly assigned in a 1:1:1:1 fashion to receive 7.5, 12.5, or 17.5 mg of HspB5 or placebo, with follow-up continuing until 48 weeks. HspB5 was administered as 3 bimonthly intravenous injections given at the beginning of the study (week 0), and at week 8 and week 16. At baseline, among the 4 study arms, the mean Extended Disability Severity Scale (EDSS) score ranged from 3.13 to 3.81, the mean number of relapses over the past 2 years from 1.75 to 2.25, and the mean time since the last relapse from 3.32 to 4.23 months.
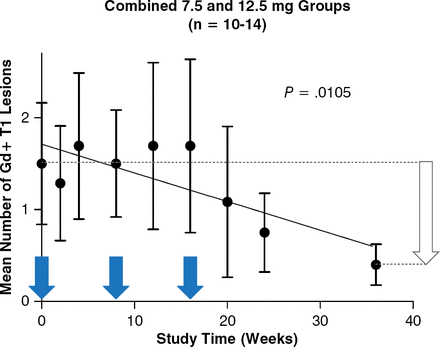
In patients who received HspB5, there was a trend toward a decrease in Gd+ T1 lesions in all HspB5 arms, as determined by magnetic resonance imaging (MRI), with the 7.5-mg dose of HspB5 resulting in a significant reduction (P = .017) over 36 weeks. When the data from the 7.5-mg and 12.5-mg HspB5 groups were combined, the MRI lesion load decreased by 75% at 36 weeks (P = .0105; Figure 1) compared with the placebo. After the last dose of HspB5 administered at 16 weeks, the reduction in MRI lesion load continued for 20 weeks. In addition, there was a similar reduction in the frequency of clinical relapses over the 36 weeks among the treatment arms. All doses of HspB5 were safe and well tolerated.
Effect of HspB5 Injection on MRI Lesion Load in RRMS
HspB5, alpha B-crystallin; Gd+, gadolinium-enhancing; MRI, magnetic resonance imaging; RRMS, relapsing-remitting multiple sclerosis.
Reproduced with permission from JM van Noort, MD.
In conclusion, the results of this trial suggest that low-dose HspB5 is safe and well tolerated and may be effective in reducing MRI lesions and clinical relapses in patients with RRMS.
- © 2014 MD Conference Express®