Summary
ALTER0302, a randomized double-blind controlled phase 2 trial, examined the efficacy and safety of anlotinib, a multitargeted tyrosine kinase inhibitor, as third-line therapy for Chinese patients with advanced non–small cell lung cancer. Progression-free survival and objective response rates were increased in all subgroups of patients treated with anlotinib with no associated serious adverse events.
- non–small cell lung cancer
- refractory advanced non–small cell lung cancer
- anlotinib
- progression-free survival
- NCT01924195
- ALTER0302
- third-line chemotherapy
- oncology clinical trials
Lung cancer, approximately 85% of which is non–small cell lung cancer (NSCLC), is the leading cause of cancer-related mortality worldwide, with an overall 5-year survival rate of 14% for stage IIIA and 1% for stage IV [American Cancer Society. http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-survival-rates. Accessed April 22, 2015]. In the past 30 years, mortality from lung cancer in China has increased by 465%, and lung cancer has been the main cause of death in urban populations [She J et al. Chest. 2013; Wen C, Dehnel T. Lancet Oncol. 2011].
Baohui Han, MD, PhD, Shanghai Chest Hospital, Shanghai, China, and colleagues examined the efficacy and safety of anlotinib—a multitargeted tyrosine kinase inhibitor, with targets that include vascular endothelial growth factor receptors 2 and 3—as third-line therapy for refractory advanced NSCLC in the randomized double-blind placebo-controlled phase 2 trial ALTER0302 [Han B et al. Ann Oncol. 2015]. The primary end point for this study was progression-free survival (PFS), while the secondary end points included objective response rate (ORR), overall survival, and safety.
Prof Han and colleagues enrolled 117 patients (aged ≥ 18 years) from 13 hospitals in China with histologically confirmed metastatic advanced NSCLC who had progressed after first- and second-line chemotherapy; had adequate hematologic, renal, and liver function; and had an ECOG PS of 0 or 1. Patients with small cell lung cancer or those with a history of hemoptysis or symptomatic brain metastases were excluded. Patients were randomized 1:1 to either anlotinib (n = 60; 12 mg/d, orally, day 1–14 every 3 weeks) or placebo (n = 57; 0 mg/d, orally, day 1–14 every 3 weeks) until disease progression, unacceptable toxicity, withdrawal of patient consent, or death.
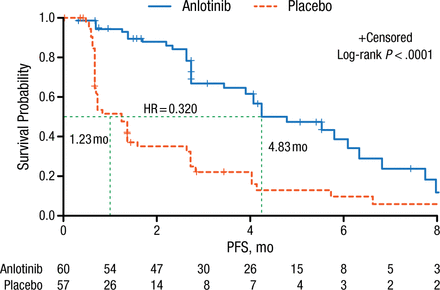
Baseline characteristics of patients stratified by age, sex, smoking history, ECOG PS, histology, stage, and number of metastases were comparable between patients receiving anlotinib and placebo. The majority of the patients had adenocarcinoma with an ECOG PS of 1, stage IV NSCLC, and > 3 metastatic lesions. Prof Han reported that PFS, the primary end point of this study, was significantly increased from 1.23 months in the placebo arm to 4.83 months in the anlotinib arm (log-rank P < .0001; Figure 1). This survival benefit was observed in all patient-stratified subgroups.
Comparison of PFS: Anlotinib vs Placebo Treatment Arms
PFS, progression-free survival.
Reproduced with permission from B Han, MD, PhD.
Although overall survival data have yet to accrue, secondary end points of this study, including ORR, extended the observed beneficial effects seen with anlotinib. Patients in the anlotinib arm exhibited a 10.00% ORR compared with 0% in the placebo arm (P < .027), and the disease control rate was reported in 83.33% of anlotinib-treated patients vs 31.58% in those treated with placebo (P < .0001). Although no serious adverse events (AEs) were noted in this study, AEs were increased from 70.2% in patients receiving placebo to 91.7% in those receiving anlotinib (P = .004). Grade 3 and 4 AEs were 15.8% in the placebo arm and 26.7% in the anlotinib arm (P = .1797) and included hypertension, hand-foot syndrome, and thyroglobulin. Based on the results of this clinical trial, Prof Han and colleagues concluded that anlotinib has significant PFS benefit as a third-line therapy in this selected Chinese population of patients with refractory advanced NSCLC with no associated serious AEs.
- © 2015 SAGE Publications