Summary
The antibacterial agent tedizolid is effective against Gram-positive pathogens, including resistant strains of methicillin-resistant Staphylococcus aureus. In the ESTABLISH-1 and -2 trials, it was noninferior to linezolid for treating acute bacterial skin and skin structure infections; this remained true in 3 pooled analyses of these studies, regardless of route of administration, infection type, or whether a causative pathogen was identified at baseline.
- acute bacterial skin and skin structure infections
- ABSSSI
- tedizolid
- linezolid
- ESTABLISH-1
- ESTABLISH-2
- efficacy
- safety
- lower extremity
- route of administration
- baseline pathogens
- antibacterial
- bacterial infections
- infectious diseases clinical trials
Three presentations reported similar efficacy and safety for tedizolid vs linezolid in patients with acute bacterial skin and skin structure infections (ABSSSIs) regardless of route of administration, type of infection, or whether a causative pathogen was identified at baseline. The reports were based on pooled data from 2 phase 3 clinical trials, ESTABLISH-1 and ESTABLISH-2 [Shorr AF et al. Antimicrob Agents Chemother. 2015]. Both trials were randomized, double-blind, multicenter trials that demonstrated the noninferiority of tedizolid (200 mg once daily for 6 days) to linezolid (600 mg twice daily for 10 days) in patients with ABSSSIs. Patients in ESTABLISH-1 [Prokocimer P et al. JAMA. 2013] received only oral drug, while ESTABLISH-2 [Moran GJ et al. Lancet Infect Dis. 2014] patients received 2 or more intravenous doses, after which they could be switched to oral doses.
For the pooled analysis, the primary study end point established by the FDA was early clinical response (≥ 20% reduction in lesion area at 48 to 72 hours compared with baseline). The primary end point required by the European Medicines Agency was the investigator’s assessment of clinical response at post-therapy evaluation (PTE). Secondary end points included clinical response at end-of-therapy (EOT) and results of microbiological efficacy analyses obtained from the primary ABSSSI site at baseline. Safety evaluations included an assessment of treatment-emergent adverse events (TEAEs). Early assessment occurred 48 to 72 hours post treatment, EOT assessment at days 11 to 13, and PTE at days 18 to 25.
Patients (aged ≥ 18 years in ESTABLISH-1 and ≥ 12 years in ESTABLISH-2) with an ABSSSI (cellulitis or erysipelas, major cutaneous abscess, or wound infection) that had a lesion area of ≥ 75 cm2, and ≥ 1 regional or systemic sign of suspected infection documented to be associated with a gram-positive pathogen, were included in the studies. There were 664 patients in the tedizolid-treated group and 669 in the linezolid group.
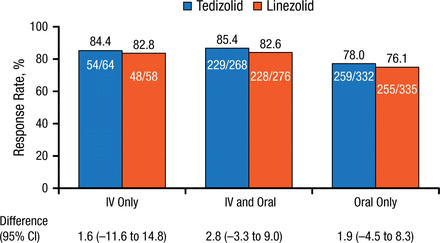
Using the pooled data, Carisa De Anda, PharmD, Merck & Co, Inc, San Diego, California, USA, reported that there were no clinically meaningful differences in efficacy or adverse event (AE) rates between the intravenous-only, intravenous and oral, and oral-only groups following tedizolid or linezolid administration. Baseline demographics and clinical characteristics were similar between treatment groups. Lesion area was greater in the intravenous-only group but similar between drug treatment groups. More patients in the intravenous-only group had a lesion surface area > 300 cm2. Methicillin-resistant Staphylococcus aureus (MRSA) infection rates were higher in the oral-only group. Early clinical response rates were similar for the 3 routes of administration and drug treatments, and ranged from 85.4% to 76.1%; differences between treatment groups were small (Figure 1).
Early Clinical Response Rates by Route of Administration
IV, intravenous.
Reproduced with permission from C De Anda, PharmD.
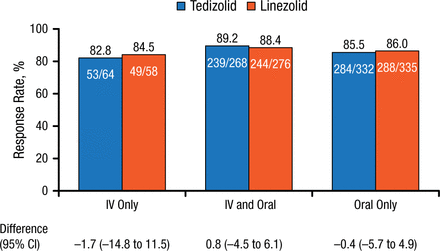
Similar values were seen for clinical responses at PTE, but with an even smaller range (82.8% to 89.2%) and treatment differences (Figure 2).
Clinical Response Rates by Route of Administration at Post-therapy Evaluation
IV, intravenous.
Reproduced with permission from C De Anda, PharmD.
There were no clinically meaningful differences in the rates of TEAEs or serious TEAEs based upon route of administration of tedizolid or linezolid. There were few drug-related TEAEs or TEAEs leading to discontinuation. Early and late clinical treatment response rates were high in ABSSSI patients, while the rates were similar regardless of route of administration or use of tedizolid vs linezolid.
Using the same pooled data, Warren Joseph, DPM, Roxborough Memorial Hospital, Philadelphia, Pennsylvania, USA, reported that once-daily (6 days) use of tedizolid had efficacy similar to 10 days of twice-daily linezolid for the treatment of lower-extremity ABSSSIs. Lower-extremity (mostly in the lower leg) ABSSSIs were seen in 270 patients in the tedizolid arm (270 of 664; 40.7%) and 282 patients in the linezolid arm (282 of 669; 42.2%). Baseline causative pathogens were isolated in 129 of 270 tedizolid and 145 of 282 linezolid patients; the majority were gram-positive aerobes (mainly S aureus and Streptococcus pyogenes). Cellulitis/erysipelas, wound infections, and cutaneous abscess were documented in 58.0%, 25.9%, and 16.1%, respectively, of patients with lower-extremity ABSSSIs.
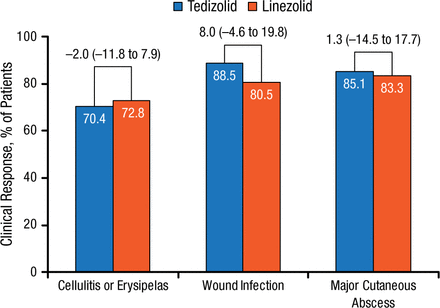
Early clinical responses were similar between treatment arms and across lower-extremity ABSSSI types (cellulitis/erysipelas, wound infections, and major cutaneous abscess). However, in both treatment groups, response rates were lower for cellulitis compared with wound infection and major cutaneous abscess (Figure 3).
Early Clinical Response by ABSSSI: Type of Infection in Lower Extremity
Values above brackets indicate treatment difference (95% CI).
ABSSSI, acute bacterial skin and skin structure infection.
Reproduced with permission from W Joseph, DPM.
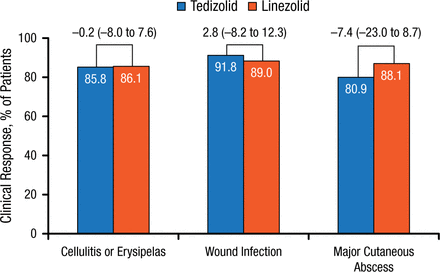
Similar findings were reported at PTE. No differences in rates of lower-extremity ABSSSI types were noted in postclinical responses between the tedizolid and linezolid treatment groups (Figure 4).
Post-therapy Evaluations by ABSSSI: Type of Infection in Lower Extremity
Values above brackets indicate treatment difference (95% CI).
ABSSSI, acute bacterial skin and skin structure infection.
Reproduced with permission from W Joseph, DPM.
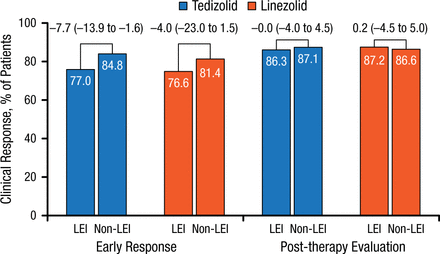
Response rate differences were noted between patients with lower-extremity ABSSSIs (5% to 8%) and those with ABSSSIs in non–lower-extremity locations for early clinical responses but not for PTE responses (Figure 5).
Early and Post-therapy Response Rates in Patients With Lower- and Non–Lower-Extremity Locations
Values above brackets indicate treatment difference (95% CI).
LEI, lower-extremity infection.
Reproduced with permission from W Joseph, DPM.
No AE rate differences were noted between treatment groups (tedizolid, 36.8%; linezolid, 39.8%).
The purpose of the study conducted by Taylor Sandison, MD, Merck & Co, Inc, San Diego, California, USA, was to compare the efficacy of tedizolid in patients with ABSSSIs in whom no pathogen was isolated at baseline with that in patients who did have a confirmed pathogen. Using the same pooled data, tedizolid appeared to be an effective option for the treatment of ABSSSIs regardless of whether a causative pathogen was identified at baseline.
Of the 664 patients randomized to tedizolid, 258 did not have a pathogen isolated at baseline. Microbiologic assessments were attempted in 207 patients with inconclusive results; in 51 patients, microbiologic assessments were not attempted. This latter group of patients had substantially larger lesion areas. Most infections were located in the lower extremities (54.7%) and diagnosed as cellulitis/erysipelas (76%).
Tedizolid remains an effective treatment regardless of whether a pathogen had been isolated at baseline (Table 1).
Clinical Responses in Patients With and Without Baseline Pathogen Isolation Treated With Tedizolid
There were no differences in AEs, TEAEs, drug-related TEAEs, serious TEAEs, or TEAEs leading to discontinuation between tedizolid-treated patients regardless of the identification of a baseline pathogen. The most frequent TEAEs in patients with unknown pathogens were nausea (8.5%), headache (5.8%), and diarrhea (4.3%). Nausea (7.9%), abscess (7.7%), and headache (6.4%) were the most common TEAEs in patients with known baseline pathogens.
Tedizolid was generally well tolerated in patients with ABSSSIs regardless of whether a causative pathogen was identified, the route of administration, or the location of the infection. Only rarely was treatment discontinued due to AEs. Overall, the most common TEAEs were nausea, headache, diarrhea, vomiting, and abscess. Tedizolid has a high bioavailability and, with its route of administration variability, may allow for shorter hospitalizations, fewer hospitalization-related complications, and cost savings.
- © 2015 SAGE Publications