Summary
A novel antibacterial agent, ceftolozane/tazobactam, compared with levofloxacin had higher rates of microbiological eradication in patients with hospital-acquired complicated urinary tract infections, including pyelonephritis, with a good safety profile, according to results of a European substudy of the ASPECT-cUTI study. The novel agent was more effective against 4 of the 5 most frequent pathogens and against drug-resistant pathogens.
- ceftolozane/tazobactam
- complicated urinary tract infections
- ASPECT-cUTI
- NCT01345929
- microbial pathogens
- microbiological eradication, infectious diseases clinical trials
- bacterial infections
Antimicrobial resistance to gram-negative pathogens is increasing. Hospital-acquired urinary tract infections (UTIs) are increasingly resistant to the antibiotics used to treat them, including the fluoroquinolones [Tandogdu Z et al. World J Urol. 2014]. Nonetheless, fluoroquinolones, including high-dose levofloxacin, are recommended as first-line therapy in clinical guidelines and remain the most widely used antibacterials to treat complicated urinary tract infection (cUTI) and pyelonephritis.
Ceftolozane/tazobactam (TOL/TAZ) is a novel cephalosporin combined with a β-lactamase inhibitor, and it has been shown to have in vitro activity against Pseudomonas aeruginosa and gram-negative pathogens, including most extended-spectrum β-lactamase (ESBL)–positive strains. This fixed-dose combination (in a 2:1 ratio) has been approved by the FDA to treat complicated intraabdominal infections and cUTIs, including pyelonephritis; an application has been submitted to the European Medicines Agency for these indications.
Florian M. Wagenlehner, MD, PhD, Justus-Liebig University, Giessen, Germany, presented findings from a European subgroup analysis of the ASPECT-cUTI trial [Wagenlehner FM et al. Lancet. 2015] that indicated that TOL/TAZ vs levofloxacin was safe and effective for the treatment of cUTIs, including pyelonephritis.
The ASPECT-cUTI trial was a double-blind phase 3 noninferiority trial conducted in 209 centers in 25 countries, and the results of the main study have been reported [Wagenlehner FM et al. Lancet. 2015]. The trial randomized men and women aged ≥ 18 years who were hospital inpatients between July 2011 and September 2013 in a 1:1 ratio to receive intravenous TOL/TAZ 1.5 g every 8 hours (n = 543) or intravenous high-dose levofloxacin 750 mg QD (n = 540) for 7 days.
The inclusion criteria included presence of pyuria, ≥ 2 clinical signs or symptoms of pyelonephritis or cUTI, and a pretreatment baseline urine culture specimen obtained within 36 hours of the first dose of the study drug. Patients were excluded if they received a nonstudy antibiotic within 48 hours of the baseline urine specimen or had renal impairment (a creatinine clearance < 30 mL/min).
The primary end point of the ASPECT-cUTI trial was the composite of microbiological eradication (ME) and clinical cure at the test-of-cure visit 5 to 9 days after the end of therapy in the patients who were microbiologically evaluable (modified intention-to-treat population). The main study found that TOL/TAZ was noninferior to levofloxacin for the primary end point (76.9% vs 68.4%). While the study was not powered to test superiority, the results indicated that TOL/TAZ was also superior to levofloxacin. The mean age of the patients was 47 years; 72% were women; and about 82% had pyelonephritis at study entry.
The European subgroup analysis was conducted to evaluate the primary end point, outcomes in patients with drug-resistant pathogens, and the safety of TOL/TAZ in the patients enrolled in Europe. Of the 812 patients, 544 qualified for the microbiologically evaluable population; 269 had been randomized to TOL/TAZ and 275 to levofloxacin. Demographic and baseline characteristics for the microbiologically evaluable population were similar between the 2 treatment groups.
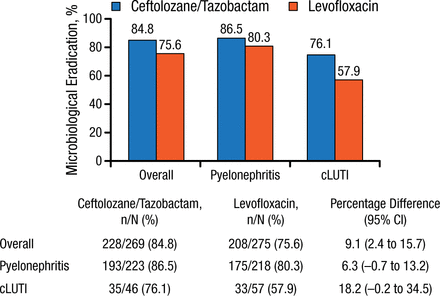
The ME rate for the main study was 84.7% with TOL/TAZ and 75.4% with levofloxacin (9.4% difference; 99% CI, 1.5% to 17.1%). In the European analysis, the ME rates for the overall cohort and for patients with pyelonephritis were similar to that in the main study and were slightly lower in the patients with cUTI (Figure 1).
Microbiological Response by Diagnosis in European Analysis
cLUTI, complicated lower-urinary tract infection.
Reproduced with permission from FM Wagenlehner, MD, PhD.
At baseline, the most frequent organism was Escherichia coli, in 78.6% of patients in the main study and 76.5% in the European subgroup; of these patients, 13% and 10%, respectively, were considered to be E coli ESBL producers. TOL/TAZ compared with levofloxacin had higher rates of ME against E coli (90% vs 78.6%), Klebsiella pneumoniae (81% vs 55%), Proteus mirabilis (100% vs 72.7%), and Pseudomonas aeruginosa (83.3% vs 45%), while it was the opposite for Enterobacter cloacae (33.3% vs 100%). TOL/TAZ had substantial rates of ME against drug-resistant pathogens compared with levofloxacin (Table 1).
Efficacy Against Drug-Resistant Pathogens
The incidence of adverse events (AEs) and serious AEs was similar between the TOL/TAZ group (27.5% and 2.0%) and the levofloxacin group (26.5% and 2.2%, respectively). More patients discontinued the study drug because of an AE in the levofloxacin group (2.0%) vs the TOL/TAZ group (1.0%).
- © 2015 SAGE Publications