Summary
Pooled data from RECLAIM 1 and 2 found that a new combination of antibiotics, incorporating ceftazidime plus avibactam plus metronidazole, was noninferior to meropenem in patients with complicated intra-abdominal infections, with no known safety signals. At the dose studied, the combination was less effective than meropenem to patients with renal impairment at baseline; however, new dosing recommendations are likely to remediate this.
- ceftazidime
- avibactam
- metronidazole
- meropenem
- intra-abdominal infections
- renal impairment
- resistance
- RECLAIM 1 and 2
- infectious diseases clinical trials
- bacterial infections
The growing prevalence of third-generation cephalosporin-resistant Enterobacteriaceae and Escherichia coli isolates throughout the world has caused an increase in the utilization of carbapenems. This has led to a resultant surge in carbapenem resistance and has presented an unmet need for antibiotics that will decrease the reliance on carbapenems for treating these infections.
John E. Mazuski, MD, Washington University School of Medicine, St Louis, Missouri, USA, presented pooled data on 2 identical phase 3 studies: RECLAIM 1 [NCT01499290] and RECLAIM 2 [NCT01500239]. The 2 studies investigated the safety and efficacy of ceftazidime-avibactam (CAZ-AVI) plus metronidazole (MTZ) compared with meropenem (MER) in treating complicated intra-abdominal infections.
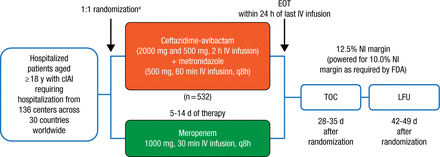
RECLAIM 1 and 2 originally enrolled 1149 adults with a diagnosis of complicated intra-abdominal infection from 30 countries between March 2012 and April 2014. Patients (n = 1066) were then randomized on a 1:1 basis to receive either CAZ-AVI plus MTZ (n = 532) or MER (n = 534) for 5 to 14 days (Figure 1). With agreement from both the European Medicines Agency and the FDA, the 2 studies were subsequently combined to form 1 global phase 3 study and analyzed using a single pooled data set. The primary end point of the trial was the clinical cure rate at the test-of-cure visit 28 to 35 days following randomization.
Study Design: RECLAIM 1 and RECLAIM 2
APACHE, Acute Physiology and Chronic Health Evaluation; cIAI, complicated intra-abdominal infection; EOT, end of treatment; IV, intravenous; LFU, late follow-up; NI, noninferiority; TOC, test of cure.
aStratified by baseline severity of disease (APACHE II score) and region.
Reproduced with permission from JE Mazuski, MD.
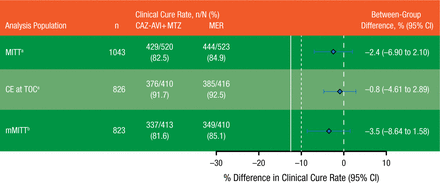
Noninferiority was assessed in the modified intention-to-treat (MITT; n = 1043) and clinically evaluable (n = 826) populations for the European Medicines Agency and the microbiologically modified intention-to-treat (mMITT) (n = 823) population for the FDA. The level of noninferiority was determined to be met if the lower limit of the 95% confidence interval for the between-group difference was > −12.50%. Adverse events (AEs) and serious AEs, including significant laboratory findings, were compared between groups in the safety population (n = 1058).
Clinical cure rates at test-of-cure visit in the MITT, clinically evaluable, and mMITT populations are summarized in Figure 2. Based on the preset definitions of inferiority, CAZ-AVI plus MTZ was noninferior to MER in all populations. In the mMITT population, CAZ-AVI plus MTZ showed clinical activity in patients infected with CAZ-resistant pathogens, with a clinical cure rate of 83.0% vs 85.9% in patients receiving MER. The treatment difference in this subgroup was −3.0% (95% CI, −17.9% to 10.6%).
Primary Efficacy Results
Solid line represents sponsor prespecified noninferiority margin of −12.5% for the lower limit of the 95% CI. Dashed line represents FDA requirement of −10%.
CAZ-AVI + MTZ, ceftazidime-avibactam plus metronidazole; CE, clinically evaluable; MER, meropenem; MITT, modified intention to treat; mMITT, microbiologically modified intention to treat; TOC, test of cure.
aEuropean Medicines Agency co–primary analysis population.
bFDA primary analysis population.
Reproduced with permission from JE Mazuski, MD.
The rate of AEs with CAZ-AVI plus MTZ was 45.9%, compared with 42.9% with MER, with serious AE rates of 7.9% and 7.6%, respectively. The most frequently reported AEs following treatment with CAZ-AVI plus MTZ were diarrhea, nausea, vomiting, and fever.
Prof Mazuski highlighted a specific subgroup of patients with moderate renal impairment at baseline. The clinical cure rates in these patients were lower in the CAZ-AVI plus MTZ group compared with the MER group (48.8% vs 74.4%; between-group percentage, −25.6; 95% CI, −44.53 to −4.78). However, because approximately two-thirds of these patients showed rapid improvement in creatinine clearance within 48 to 72 hours of dosing, the lower cure rates may reflect underdosing of the CAZ-AVI plus MTZ group in the first critical days of the study. According to Prof Mazuski, new dosing adjustments are forthcoming for patients with moderate renal impairment.
In summary, CAZ-AVI plus MTZ was noninferior to MER in the treatment of patients with complicated intra-abdominal infection and had a safety profile consistent with the known profiles of CAZ and MTZ. The combination therapy produced high response rates against key pathogens and against ceftazidime-resistant pathogens.
- © 2015 SAGE Publications