Summary
In the past 30 years, hepatitis C virus therapy has progressed from toxic regimens with low efficacy that included injectable IFN-alfa to the present highly effective interferon-free oral regimens with low toxicity. Implementation of risk reduction policies and national control strategies is needed to eradicate hepatitis C virus.
- hepatitis C virus
- interferon-free strategies
- HCV genotypes
- direct-acting antiviral drugs
- screening
- epidemiology
- global control
- HCV eradication
- emerging therapies
- infectious diseases screening & prevention
- viral infections
Hepatitis C virus (HCV) affects between 130 and 150 million individuals worldwide and is associated with about 350 000 to 500 000 deaths annually [WHO. http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed May 6, 2015]. Although antiviral therapies can cure HCV, access to diagnosis and treatment is low.
Interferon (IFN)-free strategies are proving highly effective for curing infections. With these treatment strategies, Jean-Michel Pawlotsky, MD, PhD, Henri Mondor Hospital, University of Paris-Est, Créteil, France, predicted that HCV infection will become a rare disease in 5 to 10 years, at least in high-income countries that implement a national control strategy.
HCV has been classified into 7 genotypes and 67 subtypes [Smith DB et al. Hepatology. 2014]. Genotype 1 subtypes 1a and 1b are the most common worldwide and account for about 60% of global infections [WHO. http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index2.html. Accessed May 6, 2015].
According to Prof Pawlotsky, although HCV is an important public health problem, it is also the only chronic viral infection in humans that is curable. According to the latest European Association for the Study of the Liver (EASL) recommendations for treatment of HCV [EASL. J Hepatol. 2015], a cure is evidenced by undetectable levels of HCV RNA 12 and 24 weeks after the end of treatment (sustained virologic response [SVR] 12 and SVR24).
Over the last 30 years, there has been great progress in the treatment of HCV genotype 1 (Table 1) [Pawlotsky JM et al. J Hepatol. 2015]. Early therapy using injectable IFN-alfa was associated with an SVR rate of only about 10%. This rate has increased to about 45% following the introduction of pegylated IFN and the addition of ribavirin (RBV). Most recently, the development of direct-acting antiviral (DAA) drugs has led to increased treatment success with a shorter course of treatment. As of 2014, oral treatment strategies free of IFN have achieved SVR response rates of 93% to 100% with as little as 12 weeks of therapy.
SVR Rates for HCV Genotype 1 With Successive Treatment Strategies
New DAA drugs target specific steps in the HCV life cycle that result in disruption of viral replication and infection. Targets for viral inhibition include HCV polyprotein processing, replication, assembly, and release. There are 4 classes of DAA drugs: nonstructural proteins 3/4A (NS3/4A) protease inhibitors (PIs), nonstructural proteins 5B (NS5B) nucleoside polymerase inhibitors, NS5B nonnucleoside polymerase inhibitors, and nonstructural proteins 5A (NS5A) inhibitors.
According to the EASL recommendations, therapy should be considered for treatment-naïve and treatment-experienced patients with compensated or decompensated chronic liver disease related to HCV [EASL. J Hepatol. 2015]. Treatment should be prioritized based on clinical assessment. IFN-free regimens are recommended because of their virological efficacy, ease of use, and tolerability in HCV and HIV-coinfected patients with and without cirrhosis. Prior treatment experience, HCV genotype, severity of disease, comorbidities, pharmacokinetic profile, and drug-drug interactions play a role in treatment selection.
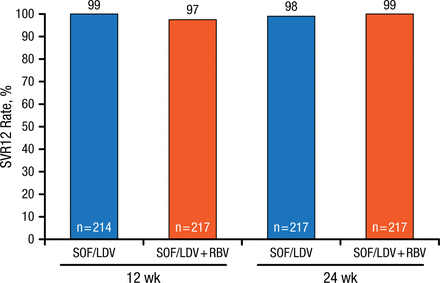
In one study in HCV patients with genotype 1 infection, once-daily ledipasvir (LDV; NS5A inhibitor)/sofosbuvir (SOF; NS5B nucleoside polymerase inhibitor) with or without RBV in a single tablet for 12 or 24 weeks was highly effective in previously untreated patients (Figure 1) [Afdhal N et al. N Engl J Med. 2014].
SOF/LDV With or Without RBV Effective Treatment for Patients With Genotype 1 HCV
HCV, hepatitis C virus; LDV, ledipasvir; RBV, ribavirin; SOF, sofosbuvir; SVR12, sustained virologic response at 12 weeks.
Source: Afdhal N et al. N Engl J Med. 2014.
Reproduced with permission from JM Pawlotsky, MD, PhD.
Similar results were reported for treatment-experienced HCV patients with compensated cirrhosis [Reddy KR et al. Hepatology. 2015]. In previously untreated patients with HCV genotype 1 infection and no cirrhosis, a 12-week multitargeted regimen consisting of a single-tablet coformulation (NS3/4A PI paritaprevir with ritonavir [paritaprevir-ritonavir], the NS5A inhibitor ombitasvir, and the NS5B nonnucleoside inhibitor dasabuvir) with RBV was highly effective (SVR12, 95% to 98%) and associated with low rates of treatment discontinuation [Feld JJ et al. N Engl J Med. 2014].
The same oral combination regimen of paritaprevir/ritonavir plus ombitasvir plus dasabuvir, and RBV, for 12 or 24 weeks resulted in high SVR rates among patients coinfected with HCV genotype 1 and HIV-1 whether treated for 12 or 24 weeks [Sulkowski MS et al. JAMA. 2015]. Other studies have reported similar high SVR rates with different combinations of DAA drugs in post–liver transplant patients [Kwo PY et al. N Engl J Med. 2014], in patients on opioid substitution therapy [Lalezari J et al. J Hepatol. 2015], and in treatment-naïve or treatment-experienced patients [Lawitz E et al. Lancet. 2014].
SOF plus RBV or SOF plus daclatasvir with or without RBV are options in Europe for treating HCV with genotype 2 or 3 [EASL. J Hepatol. 2015]. In published trials, overall rates of SVR12 were higher in patients with genotype 2 than in those with genotype 3 infection and those without cirrhosis compared with those who had cirrhosis [Lawitz E et al. Hepatology. 2015; Nelson DR et al. Hepatology. 2015; Jacobson M et al. N Engl J Med. 2013; Lawitz E, Gane EJ. N Engl J Med. 2013].
Genotype 4 virus accounts for about 13% of global HCV infections [Hézode C et al. Lancet. 2015]. Ombitasvir and paritaprevir/ritonavir, given with or without RBV to HCV patients, had high SVR rates at 12 weeks in treatment-naïve and experienced patients with HCV genotype 4 infections. HCV genotype 4 can also be effectively treated with a single-tablet formulation of SOF and LDV (SOF/LDV), achieving an SVR12 of 95% [Kapoor R et al. AASLD 2014 (abstr 240)], and genotype 6 HCV responds to SOF/LDV with or without RBV [Gane EJ et al. AASLD 2014 (abstr LB11)].
Several issues, such as drug-drug interactions, the timing of therapy in the pretransplant setting, treating patients with renal insufficiency, developing retreatment strategies, and possible resistance to DAAs in patients who fail treatment with DAAs, need to be addressed to achieve global treatment goals. For instance, simeprevir (an oral NS3/4A PI) is usually effective in combination with other anti-HCV drugs [Jensen DM et al. AASLD (abstr 45)]. However, baseline NS3 polymorphisms at positions associated with reduced in vitro susceptibility to simeprevir have been linked to the emergence of high-level resistance mutations and treatment failure [Lenz O et al. J Hepatol. 2015].
Prof Pawlotsky concluded that it is not feasible to eradicate an infection present in > 130 million individuals by antiviral therapy alone. Control of HCV infection should be the goal in regions that can afford it, as there are currently no HCV vaccines available. For global control to be effective, the transmission of HCV must be prevented, and individuals infected with HCV need to be identified by appropriate screening practices.
Under the 2001 French HCV screening strategy, all blood donors and individuals with HCV risk factors (eg, drug users, hemodialysis patients, HIV-positive individuals, those having prison records, and individuals with alanine aminotransferase elevations) should be screened. Tools for screening include point-of-care or dried blood spot tests. The focus should be on seeking out undiagnosed, seropositive individuals. Despite being curable, major issues remain regarding cost of care and access to care in low- and middle-income areas.
- © 2015 SAGE Publications