Summary
A 3-drug (3D) regimen of ABT-450, co-dosed with ritonavir, ombitasvir and dasabuvir has been given alone or in combination with ribavirin (RBV) and studied in 6 phase 3 trials of more than 2700 patients. The 3D regimen is safe and effective in patients infected with hepatitis C virus (HCV) genotype 1a with or without cirrhosis. However, efficacy of this treatment may be variably influenced by HCV genotype subtype, treatment experience (naïve vs experienced), and stage of fibrosis (early fibrosis vs cirrhosis). This article discusses an analysis of the SAPPHIRE-I and -II, PEARL-IV, and TURQUOISE-II trials to examine the impact of RBV in noncirrhotic patients and treatment duration in cirrhotic patients.
- Hepatology Clinical Trials Liver Conditions
- Viral Infections
- Hepatology Clinical Trials
- Hepatology
- Liver Conditions
- Viral Infections
A 3-drug (3D) regimen of ABT-450, an NS3/4A protease inhibitor, co-dosed with ritonavir, ombitasvir, an NS5A inhibitor, and dasabuvir, a non-nucleoside NS5B polymerase inhibitor has been given alone or in combination with ribavirin (RBV) and studied in 6 phase 3 trials of more than 2700 patients. The 3D regimen is safe and effective in patients infected with hepatitis C virus (HCV) genotype 1a with or without cirrhosis. However, efficacy of this treatment may be variably influenced by HCV genotype subtype, treatment experience (naïve vs experienced), and stage of fibrosis (early fibrosis vs cirrhosis).
In this analysis, data from 1058 patients with HCV genotype 1a from 4 phase 3 trials were pooled to examine the impact of RBV in noncirrhotic patients and treatment duration in cirrhotic patients. The studies were SAPPHIRE-I and -II [Feld JJ et al. N Engl J Med. 2014; Zeuzem S et al. N Engl J Med. 2014], PEARL-IV [Ferenci P et al. N Engl J Med. 2014], and TURQUOISE-II [Poordad F et al. N Engl J Med. 2014] (Table 1). Gregory Everson, MD, University of Colorado, Denver, Colorado, USA, presented the results.
The Pooled Studies
Key inclusion criteria for this study were chronic HCV infection with genotype 1a, age 18 to 70 years, and plasma HCV RNA > 10 000 IU/mL. Key exclusion criteria were infection with hepatitis B virus or human immunodeficiency virus.
PATIENTS WITHOUT CIRRHOSIS
The 3D regimen was administered for 12 weeks with or without RBV. Overall, the rates of SVR12 with and without RBV were 96.0% (569/593) and 90.1% (182/202) (P = .004). In treatment-naïve patients, rates of SVR12 were 96.0% (403/420) and 90.1% (182/202) (P = .0 0 6), respectively. All treatment-experienced patients were given RBV, and rates of SVR12 were 94% for relapsers, 100% for partial responders, and 95.4% for null responders. RBV dose modification was required in 6.7% of RBV-treated patients, but, despite the dose modification, 97.6% of these patients achieved SVR12. In multivariable analyses, high baseline body mass index and RBV-free treatment were associated with lower likelihood of achieving SV R12 (P = .005 and P = .007, respectively). Adverse events and related discontinuations were generally mild and were more common in RBV-containing treatment.
PATIENTS WITH CIRRHOSIS
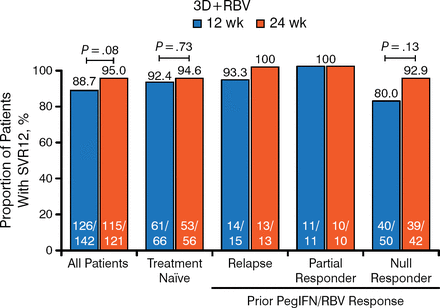
All of the patients with cirrhosis were treated with RBV, and treatment durations of 12 and 24 weeks were compared (Figure 1). The overall rates of SVR12 were 88.7% for 12 weeks and 95% for 24 weeks (P = .08). The lower SVR12 with 12 weeks was mainly evident in the treatment-experienced patients with prior null response, 80.0% versus 92.9% for 12 versus 24 weeks of treatment (P = .13). Rates of SVR12 in the treatment-naïve patients were 92.4% and 94.6% for 12 and 24 weeks of treatment, respectively.
SVR12 Rates in Cirrhotic HCV1a-Infected Treatment-Naïve and Treatment-Experienced Patients Treated for 12 or 24 Weeks
3D, 3-drug direct-acting antiviral regimen; HCV1a, hepatitis C virus genotype 1a; PegIFN, pegylated interferon; RBV, ribavirin; SVR12, sustained virologic response at week 12.
In multivariable analyses, the TT IL28B genotype and a prior null response to peginterferon/RBV therapy were associated with failure to achieve SVR12 (P = .008 and P = .009, respectively). Adverse events and event-related discontinuation were similar for both treatment durations.
This pooled analysis of 4 phase 3 trials of patients infected with HCV genotype 1a indicates that the rate of SVR in noncirrhotic patients treated with the 3D regimen may be enhanced by addition of RBV. In addition, in patients with cirrhosis, extension of 3D + RBV to 24 weeks may be warranted, especially for those who were prior null responders to peginterferon/RBV.
- © 2014 MD Conference Express®