Summary
This article presents data indicating that treatment with an all-oral fixed-dose combination of daclatasvir/asunaprevir/beclabuvir with or without the addition of ribavirin is associated with high rates of sustained viral response to treatment 12 weeks after therapy in patients with chronic compensated cirrhosis and hepatitis C virus genotype 1 infection.
- Liver Conditions Hepatology Clinical Trials
- Viral Infections
- Liver Conditions
- Hepatology
- Hepatology Clinical Trials
- Viral Infections
Andrew J. Muir, MD, MHS, Duke University School of Medicine, Durham, North Carolina, USA, presented data indicating that treatment with an all-oral fixed-dose combination of daclatasvir (DCV)/asunaprevir (ASV)/beclabuvir (BCV; DCV-Trio) with or without the addition of ribavirin (RBV) is associated with high rates of sustained viral response to treatment 12 weeks after therapy (SVR12) in patients with chronic compensated cirrhosis and hepatitis C virus (HCV) genotype 1 (GT-1) infection.
In a phase 2 trial, combination treatment with DCV/ASV/BCV was associated with SVR12 in > 92% of treatment-naïve patients with GT-1 infection and 100% of patients with GT-4 infection [Hassanien T et al. J Hepatol. 2014; Everson GT et al. AASLD 2013]. UNITY-2 [NCT01973049] was a randomized phase 3 trial that evaluated DCV/ASV/BCV as a fixed-dose regimen with and without RBV in treatment-naïve (n = 112) and treatment-experienced (n = 90) patients with HCV GT-1 infection and compensated cirrhosis. Adults with chronic HCV GT-1a or 1b infection and confirmed compensated Child-Pugh class A cirrhosis with a platelet count > 50 000/mm2, international normalized ratio < 1.7, and albumin > 3.5 g/dL were eligible to participate. Patients were randomized to DCV-Trio (DCV 30 mg/ASV 200 mg/BCV 75 mg) with or without blinded RBV and treated twice daily for 12 weeks. The key efficacy variable was SVR12 (HCV RNA < lower limit of quantification [25 IU/mL] target detected/not-detected) in the treatment- naïve and treatment-experienced patients evaluated separately.
Participants were predominately white (> 80%) men (> 60%) between 58 and 60 years of age. Between 70% and 78% of the participants were GT-1a; less than one-third had the favorable IL28B CC GT polypmorphism; 26% of patients had platelets < 100 000/mm2. Of the 90 treatment-experienced patients, 39% were prior null responders; 9% were partial responders and 18% of patients had relapsed. Nonresponse was characterized as “other” in the remaining 34%.
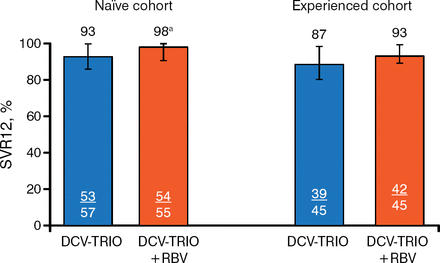
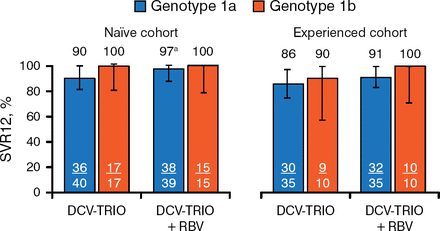
In the treatment-naïve cohort, 93% of patients receiving DCV-Trio only achieved SVR12 compared with 98% of those who received DCV-Trio plus RBV. Results were similar in the experienced cohort (87% and 93%, respectively; Figure 1) and when analyzed by GT-1 subtype (Figure 2).
SVR12 in the Modified Intention-to-Treat Population
DC V-Trio, daclatasvir/asunaprevir/beclabuvir; HCV, hepatitis C virus; LLOQ, lower limit of quantification; RBV, ribavirin; SVR12, sustained viral response to treatment 12 weeks after therapy.
aOne patient with HCV RNA < LLOQ TND at end of therapy and posttreatment week 4 had missing data at posttreatment week 12. Error bars indicate 97.5% confidence intervals. Reproduced with permission from AJ Muir, MD, MHS.
SVR12 by Genotype-1 Subtype
DCV-Trio, daclatasvir/asunaprevir/beclabuvir; HCV, hepatitis C virus; LLOQ, lower limit of quantification; RBV, ribavirin; SVR12, sustained viral response to treatment 12 weeks after therapy.
aOne patient with HCV RNA < LLOQ TND at end of therapy and posttreatment week 4 had missing data at posttreatment week 12.
Error bars indicate 97.5% confidence intervals.
Reproduced with permission from AJ Muir, MD, MHS.
No clear difference in SV12 was observed between patients who did and did not receive RBV when assessed by platelet count (< vs ≥ 100 000/mm2), sex, age, baseline HCV RNA, or IL28B GT.
Three patients had on-treatment virologic failure; all were in the treatment-experienced group. Ten patients relapsed (4 treatment-naïve patients; 6 treatment-experienced patients; Table 1). NS5A and NS3 resistance-associated variants (RAVs) did not appear to impact SV12.
Virologic Outcomes
Two patients in the DCV-Trio-only group had serious adverse events (AEs) compared with 7 patients receiving DCV-Trio plus RBV. AEs were similar to those seen in HCV trials and slightly higher among patients receiving RBV.
The all-oral fixed-dose combination of DCV/ASV/BCV with or without RBV was generally safe, well tolerated, and associated with high rates of SVR12. The addition of RBV decreased the frequency of relapse in patients with GT-1a. Baseline RAVs do not appear to impact response.
- © 2014 MD Conference Express®