Summary
The ASPECT-cIAI trial demonstrates high clinical cure rates, similar to those of meropenem, with ceftolozane/tazobactam plus metronidazole in patients with complicated intra-abdominal infections in a European population. In addition, a post hoc analysis shows similar outcomes between patients who are nonobese and obese.
- ceftolozane/tazobactam
- metronidazole
- meropenem
- complicated intra-abdominal infection
- ASPECT-cIAI
- European population
- obesity
- infectious diseases clinical trials
- emerging therapies
A major challenge in the treatment of complicated intra-abdominal infections (cIAIs), including secondary or tertiary peritonitis and cIAI associated with health care, is the potential that a resistant pathogen is responsible for the infection [Eckmann C, Shekarriz H. Eur Infect Dis. 2012]. In these settings, extended-spectrum β-lactamase (ESBL)–producing organisms and multidrug-resistant Pseudomonas are particularly prevalent. Treatment may be additionally complex in patients who are obese, as a result of obesity-associated factors such as decreased immune function or dysregulation, the presence of comorbidities, and respiratory dysfunction. Furthermore, the pharmacokinetics/pharmacodynamics of β-lactam antibiotics may be altered in patients who are obese [Pai MP, Bearden DT. Pharmacotherapy. 2007].
Two presentations reporting on different subsets of patients focused on the safety profile and efficacy of ceftolozane/tazobactam (TOL/TAZ) plus metronidazole (MTZ) in the treatment of cIAIs based on data from the ASPECT-cIAI trial [Solomkin J et al. Clin Infect Dis. 2015].
Christian Eckmann, MD, Academic Hospital of Medical University Hannover, Peine, Germany, presented data assessing TOL/TAZ plus MTZ compared with meropenem (MER) in European patients. TOL/TAZ, which is currently approved by the FDA for the treatment of complicated urinary tract infections and cIAIs, has demonstrated activity against Pseudomonas aeruginosa in vitro, including organisms with drug-resistant mechanisms [Farrell DJ et al. Antimicrob Agents Chemother. 2013]. While TOL/TAZ is active against some of the most common anaerobic pathogens encountered in cIAI, including Bacteroides fragilis, it is not active against all anaerobic pathogens; therefore, it must be used with MTZ in patients with cIAIs [Snydman DR et al. Antimicrob Agents Chemother. 2014].
In the international double-blind phase 3 ASPECT-cIAI trial, 993 adults with clinical evidence of cIAI were randomly assigned to receive TOL/TAZ 1.5 g plus MTZ 500 mg every 8 hours or MER 1 g every 8 hours intravenously for 4 to 14 days [Solomkin J et al. Clin Infect Dis. 2015]. Patients were excluded if the cIAI was managed by staged abdominal repair without closed fascia, the source control during surgery was likely inadequate, systemic antimicrobials were used to treat cIAI for > 24 hours prior to initiation of study drug, the creatine clearance was < 30 mL/min, and there was a presence of septic shock.
Patients (n = 764) enrolled in European centers were analyzed. The mean age was 51.4 years, and about 56% of patients were men. At baseline, 51.5% of patients received prior antibiotic therapy, and the mean Acute Physiology and Chronic Health Evaluation II score was 6. Major anatomic locations of the cIAI included the appendix, biliary tract, small bowel, and colon.
At the test-of-cure visit (24 to 32 days from start of therapy), the overall clinical cure rates were similar between treatment arms, with a weighted difference of 0.6 (99% CI, −3.99 to 5.13) in the clinically evaluable population. However, the clinical cure rates differed among the treatment arms according to pathogen (Table 1). TOL/TAZ plus MTZ treatment resulted in greater clinical cure rates in patients infected with Klebsiella pneumonia, ESBL-producing Klebsiella pneumoniae, and P aeruginosa compared with MER. In contrast, MER treatment resulted in higher clinical cure rates in patients infected with Enterobacter cloacae compared with TOL/TAZ treatment.
Clinical Cure Rates According to Pathogen in European Patients in ASPECT-cIAI
Treatment-emergent adverse events (TEAEs) occurred more frequently in the TOL/TAZ plus MTZ arm compared with the MER arm (36.9% vs 34.5%). Common TEAEs included nausea, vomiting, diarrhea, pyrexia, hypokalemia, and insomnia. Serious TEAEs occurred in 7% of patients in the TOL/TAZ arm and 4.7% of patients in the MER arm. In the TOL/TAZ and MER arms, 9 and 5 deaths occurred, respectively; however, the investigators determined that the deaths were due to treatment failures for unrelated conditions or were indeterminate and not associated with the study drugs. In the study, 10 (2.7%) and 7 (1.8%) of patients who received TOL/TAZ and MER, respectively, discontinued the study due to TEAEs.
In conclusion, Prof Eckmann stated that the data from the ASPECT-cIAI trial suggest that treatment of cIAI with TOL/TAZ plus MTZ resulted in high clinical cure rates, with greater success than MER for the pathogen P aeruginosa and many Enterobacteriaceae, including ESBL-positive strains.
Benjamin Miller, PharmD, Cubist Pharmaceuticals, Lexington, Massachusetts, USA, presented a post hoc analysis of the ASPECT-cIAI trial assessing clinical response in nonobese and obese patients. Of the total patients in the trial, 239 were considered obese, with a mean body mass index (BMI) of 32.9 kg/m2.
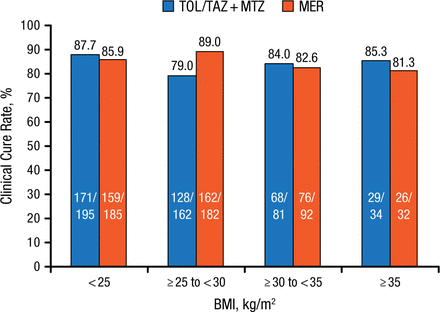
The clinical cure rates with TOL/TAZ treatment were somewhat lower vs MER in patients who were nonobese (BMI < 30; 83.8% vs 87.5%), whereas they were higher in patients who were obese (BMI ≥ 30; 84.3% vs 82.3%). In addition, TOL/TAZ treatment appeared to be least effective in patients with a mean BMI of ≥ 25 to < 30 kg/m2 (Figure 1). However, Dr Miller concluded that, overall, TOL/TAZ efficacy and safety outcomes were similar between nonobese and obese patients.
Clinical Cure Rates in ASPECT-cIAI According to BMI
BMI, body mass index; MER, meropenem; MTZ, metronidazole; TOL/TAZ, ceftolozane/tazobactam.
Reproduced with permission from B Miller, PharmD.
The most common TEAEs in obese and nonobese patients included diarrhea, nausea, vomiting, and pyrexia, with diarrhea and nausea occurring more commonly in obese patients in the TOL/TAZ plus MTX arm vs the MER arm.
To conclude, BMI had no impact on clinical outcomes in patients treated with TOL/TAZ plus MTZ. With the exception of diarrhea and nausea in the obese subset, TEAEs were comparable between the 2 treatment arms.
- © 2015 SAGE Publications