Summary
Adults with attention deficit hyperactivity disorder may benefit from an oral regimen of dasotraline, which inhibits reuptake of dopamine and norepinephrine. Deficiencies of both compounds have been associated with attention deficit hyperactivity disorder symptoms. The 4-week, randomized, double-blind, placebo-controlled trial provided proof-of-concept for dasotraline use, and should prompt further clinical trials and dose optimization.
- dasotraline
- dopamine
- norepinephrine
- ADHD
- insomnia
- psychiatry & psychology clinical trials
A 4-week, randomized, double-blind, placebo-controlled trial [Koblan KS et al. Neuropsychopharmacology. 2015] demonstrated clinically meaningful effects with dasotraline, an inhibitor of the reuptake of dopamine and norepinephrine, in the treatment of adults with attention-deficit/hyperactivity disorder (ADHD).
Symptoms of ADHD, including restlessness, impulsive behavior, forgetfulness, and distractibility, can hamper social interactions and work/school performance, and ADHD that is first evident in childhood can continue into adulthood. Drugs that increase dopamine and norepinephrine transmission have proven clinical value in the management of ADHD symptoms. Dasotraline blocks the presynaptic reuptake of dopamine and norepinephrine, increasing their levels in the brain.
This trial, conducted by Kenneth S. Koblan, PhD, Sunovion Pharmaceuticals, Marlborough, Massachusetts, USA, and colleagues, explored the potential value of dasotraline in adults with ADHD. Secondary objectives included evaluations of dasotraline safety and tolerability.
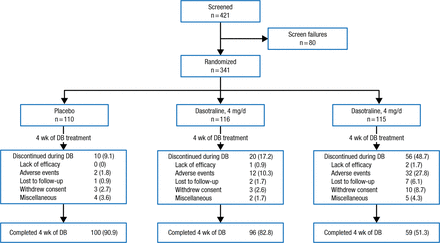
Adult outpatients aged 18 to 55 years (n = 341) with a primary diagnosis of ADHD based on DSM-IV-TR criteria were randomized 1:1:1 in a double-blind fashion to a 4-week oral regimen of dasotraline 4 or 8 mg/d, or to placebo. The treatment period was followed by a 2-week washout (Figure 1).
Study Design
Data presented in No. (%).
DB, double-blind.
Adapted by permission from Macmillan Publishers Ltd: Nerophsychopharmacology. Koblan KS et al. Dasotraline for the Treatment of Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind, Placebo-Controlled, Proof-of-Concept Trial in Adults. Advance online publication 3 June 2015; doi: 10.1038/npp.2015.124. Copyright (2015).
Inclusion criteria other than diagnosis of ADHD included prior treatment with at least one ADHD medication (stimulant or nonstimulant), and a Clinical Global Impression, Severity (CGI-S) score ≥ 4 at baseline. Exclusion criteria were history of bipolar disorder, schizophrenia, or other psychotic disorder; substance abuse/dependence in the prior 12 months, or a positive alcohol or drug screen test result during trial screening; and treatment in the preceding 6 months with specified medications. Use of zolpidem, zaleplon, and eszopiclone for insomnia management was allowed.
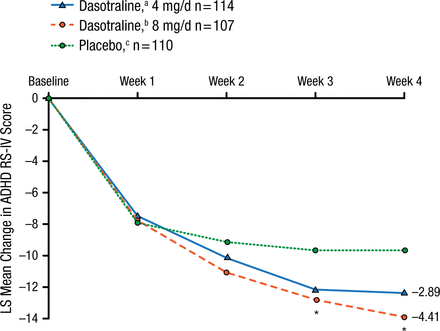
The intention-to-treat arms for dasotraline 4 mg, 8 mg, and placebo were demographically/clinically comparable at baseline. The primary outcome of mean change from baseline in the ADHD Rating Scale, version IV score at 4 weeks was reduced by dasotraline 4 and 8 mg vs placebo (Figure 2).
Primary Outcome
The primary efficacy measure of ADHD RS-IV (with adult prompts) total score was reduced by dasotraline 4 mg (adjusted P = .076) and 8 mg (adjusted P = .019) at 4 wk compared with placebo.
ADHD RS-IV, Attention Deficit Hyperactivity Disorder Rating Scale version IV; LS, least squares.
aBaseline mean, 36.8.
bBaseline mean, 36.6.
cBaseline mean, 36.7.
*P < .05.
Adapted by permission from Macmillan Publishers Ltd: Nerophsychopharmacology. Koblan KS et al. Dasotraline for the Treatment of Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind, Placebo-Controlled, Proof-of-Concept Trial in Adults. Advance online publication 3 June 2015; doi: 10.1038/npp.2015.124. Copyright (2015).
Both doses of dasotraline significantly improved the CGI-S score compared with placebo at 4 weeks (4 mg, P = .021; 8 mg, P = .013). Significant improvements in the hyperactivity/impulsivity (P = .027) and inattentiveness (P = .016) subscales were also evident with the 8 mg dose arm.
Adverse events that occurred more often in those receiving dasotraline included insomnia, decreased appetite, anxiety, nausea, and dizziness. Events leading to patient withdrawal for the 4 and 8 mg doses of dasotraline were insomnia (2.6% and 10.8%), anxiety (2.6% and 1.8%), and panic attacks (0% and 2.7%); these events did not occur in the placebo arm. The mean changes in the Insomnia Severity Index scores for both doses of dasotraline were significantly higher at weeks 1 through 4 compared with placebo.
The proof-of-concept trial demonstrated clinically meaningful benefits in adults with ADHD using dasotraline, with benefits being significantly better than placebo for the 8 mg dose. The observation of increased plasma levels of the norepinephrine metabolite dihydroxyphenylglycol with increasing dasotraline dose supports the view that the drug’s beneficial effects reflect inhibition of dopamine and norepinephrine reuptake. Further clinical trials, including dose optimization, are anticipated.
- © 2015 SAGE Publications