Summary
Acute and chronic pancreatitis are distinct pathologies requiring unique classification in order to devise the optimum prevention, treatment, and management strategies. AP is defined by a combination of abdominal pain, elevated pancreatic enzymes in serum, and radiologic features of AP. Management depends on defining severity. AP can progress into CP, a long-lasting inflammation of the pancreas that may worsen over time and can lead to organ damage.
- acute pancreatitis
- chronic pancreatitis
- organ failure
- necrotizing pancreatitis
- fluid resuscitation
- enteral feeding
- endoscopic retrograde cholangiopancreatography
- pancreas divisum
- recurrent and relapsing pancreatitis
- hereditary pancreatitis
- pancreatic cancer
- human exocrine pancreatic insufficiency
- steatorrhea
- endoscopic ultrasound
- gastroenterology procedures
In this American Gastroenterological Association State-of-the Art Lecture, Peter A. Banks, MD, Brigham and Women’s Hospital, Boston, Massachusetts, USA, and Eugene P. DiMagno, MD, Mayo Clinic, Rochester, Minnesota, USA, presented a historical overview of the landmark studies, the current management, and the future treatment direction for acute pancreatitis (AP) and chronic pancreatitis (CP). Dr Banks began with a review of the landmark studies that advanced the understanding of AP.
Because of the wide variability in presentation and clinical course, there was initially no accepted clinical classification system for AP. The first objective criteria for early identification of severe acute pancreatitis was published in 1974 [Ranson JH et al. Am J Gastroenterol. 1974], and the first computed tomography scoring system was published in 1985 [Balthazar EJ et al. Radiology. 1985]. The third major contribution to the literature was published results from a trial that used guided percutaneous aspiration to identify pancreatic infection at an early stage [Gerzof SG et al. Gastroenterology. 1987].
The Atlanta Classification Study was the first published formal classification system for AP [Bradley EL III. Arch Surg. 1993]. The next series of landmark studies classified organ failure into transient and persistent [Mofidi R et al. Br J Surg. 2006; Johnson CD, Abu-Hilal M. Gut. 2004; Buter A et al. Br J Surg. 2002] and proposed the nonsurgical treatment of severe necrotizing pancreatitis [Bradley EL III, Allen K. Am J Surg. 1991]. Later studies showed that, in some cases, infected necrosis could be treated with antibiotics [Runzi M et al. Pancreas. 2005]. The concept of extrapancreatic necrosis was introduced in 1999 [Sakorafas GH et al. J Am Coll Surg. 1999]; about 12 years later, a “moderately severe” category of AP was identified [Talukdar R et al. Pancreas. 2012]. This new category was incorporated in the 2012 revision of the Atlanta system along with a new classification for AP [Banks PA et al. Gut. 2013].
Dr Banks also reviewed some of the landmark papers focused on the management of pancreatitis. Several studies addressed the usefulness of systemic inflammatory response syndrome in the first 24 hours in defining morbidity and mortality [Singh VK et al. Clin Gastroenterol Hepatol. 2009; Mofidi R et al. Br J Surg. 2006; Buter A et al. Br J Surg. 2002], as well as studies presenting new scoring systems [Lankisch PG et al. Clin Gastroenterol Hepatol. 2009; Wu BU et al. Gut. 2008; Larvin M, McMahon MJ et al. Lancet 1989]. Other studies showed that hematocrit was an important risk factor in acute necrotizing pancreatitis [de-Madaria E et al. Clin Gastroenterol Hepatol. 2014; Wu BU et al. Pancreas. 2010; Baillargeon JD et al. Am J Gastroenterol. 1998] and that blood urea nitrogen levels were a predictor of mortality [Wu BU et al. Arch Int Med. 2011]. More work is needed in this area, as none of these tools are very accurate when it comes to predicting persistent organ failure in AP at admission and at 48 hours [Mounzer R et al. Gastroenterology. 2012].
The benefits of early fluid resuscitation and the potential benefit of lactated Ringer solution compared with saline were first reported in 2011 [Warndorf MG. Clin Gastroenterol Hepatol. 2011; Wu BU et al Clin Gastroenterol Hepatol. 2011]. However, there is still no consensus regarding the amount of fluids and when to administer. A 1997 study demonstrated the superiority of enteral feeding vs parenteral nutrition in patients with severe AP [Kalfarentzos F et al. Br J Surg. 1997], whereas a 2005 study recommended nasogastric vs nasojejunal feeding [Eatock FC et al. Am J Gastroenterol. 2005]. Most recently, the PYTHON study [Bakker OJ et al. N Engl J Med. 2014] showed no benefit of early nasoenteric tube feeding vs oral feeding in reducing infection or death after 72 hours.
There is still controversy regarding the benefit of antibiotic prophylaxis in patients with severe necrotizing pancreatitis [Dellinger EP et al. Ann Surg. 2007], and a 1997 study showed no benefit to endoscopic retrograde cholangiopancreatography in biliary pancreatitis [Fölsch UR et al. N Engl J Med. 1997].
The treatment of necrotizing pancreatitis has undergone a remarkable transition from ostomies and sump pumps of the 1970s and subtotal pancreatectomy of the 1980s to the minimally invasive approach used today. Similar advances have occurred in radiologic drainage of infected pseudocytes and infected necrosis, which have led to data suggesting that a minimally invasive step-up approach, as compared with the primary open approach, reduced the rate of the composite end point of major complications or death, as well as long-term complications [van Santvoort HC et al. N Engl J Med. 2010].
Future requirements for progress entail prevention of AP and progression to CP, improvements in detectors of severity, better treatments, more research and training, and support from professional organizations and foundations.
Dr DiMagno discussed CP. Early studies reported on the connection between pancreas divisum, AP, and CP. A later study identified abnormalities of the cystic fibrosis gene in patients with pancreas divisum as the explanation for why some individuals developed pancreatitis [Gelrud A et al. Am J Gastroenterol. 2004]. This has led to a recommendation for cystic fibrosis genetic testing when pancreas divisum and pancreatitis are present. The use of computed tomographic examination is a useful prognostic indicator of morbidity and mortality, able to reveal focal or diffuse enlargement, and fluid collection.
It is important to distinguish recurrent AP (RAP) from relapsing CP (RCP). Patients with RCP have pain lasting for days with repeated attacks of acute interstitial pancreatitis or hemorrhage pancreatitis leading to eventual destruction of the acinar and islet cells. RAP has identifiable causes (eg, gallstones, hypertriglyceridemia), does not lead to CP, and if treated, attacks can be reduced. CP can be both RCP and established CP. There is also alcoholic and idiopathic (early and late) CP, each with distinct characteristics.
Hereditary pancreatitis is a form of AP with the same painful episodes. Onset is typically between ages 5 and 23 and progressively destroys the pancreas. It can be accompanied by stones in the duct and perhaps calcification of the parenchyma. In 1996, Whitcomb reported that hereditary pancreatitis is caused by a mutation in the cationic trypsin gene mapped to chromosome 7q35. Since then, multiple genetic mutations have been associated with pancreatitis. This new genetic information will be useful for understanding interactions among environment factors and may lead to the ability to predict probability of developing pancreatitis and prevent/cure the disease.
Hereditary pancreatitis and nonhereditary CP significantly increase the risk of pancreatic cancer several decades after the diagnosis (Figures 1 and 2).
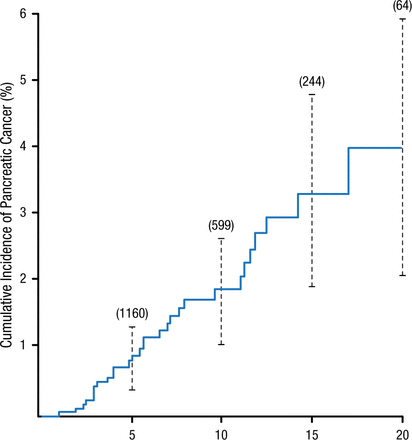
Cumulative Incidence of Pancreatic Cancer in Patients With Nonhereditary Chronic Pancreatitis
Cumulative Incidence of Pancreatic Cancer in 1552 Subjects with Chronic Pancreatitis with a Minimum of Two Years of Follow-Up.
The vertical lines represent 95 percent confidence intervals. The numbers in parentheses are the numbers of subjects at risk. One additional case of cancer developed after 25 years of follow-up.
From N Engl J Med, Lowenfels AB et al, Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group, Volume No. 328, Page No. 1433-1437. Copyright © (1993) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
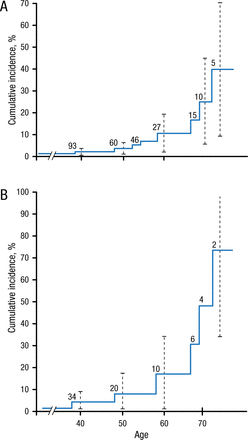
Cumulative Incidence of Pancreatic Cancer in Patients With Hereditary Pancreatitis
A) Cumulative incidence (%) of pancreatic cancer since birth in entire cohort of 246 patients with hereditary pancreatitis. B) Cumulative incidence (%) of pancreatic cancer in 105 patients with a paternal inheritance pattern. Vertical dotted lines 4 95% confidence intervals, and numbers within graph indicate patients at risk.
Lowenfels AB et al. Hereditary Pancreatitis and the Risk of Pancreatic Cancer. J Natl Cancer Inst. 1997; 89(6): 442-446. By permission of National Cancer Institute.
Human exocrine pancreatic insufficiency due to lipase output < 10% of normal can lead to steatorrhea and abdominal pain. A postprandial lipase concentration 90 000 USP Units (> 5% normal) is effective in abolishing steatorrhea. Porcine lipase is being tested as a future management strategy for pancreatic insufficiency.
Pancreatitis is diagnosed by pancreatic function testing, imaging, and endoscopic ultrasonography. Endoscopic ultrasonography combined with pancreatic function testing or measuring markers in pancreatic juice is 1 approach for detecting CP.
Dr DiMagno concluded that recommendations for preventing and treating CP include reduction of environmental risk factors, healthy living, and modification of gene-associated mutations.
- © 2015 SAGE Publications