Summary
Results of the ALLY 2 trial involving more than 200 patients co-infected with HIV/HCV shows the efficacy and safety of the combined 12-week use of daclatasvir and sofosbuvir, with sustained virologic response for 12 weeks of 97% after a 12-week treatment. The benefit for HCV infection does not compromise the ongoing antiretroviral therapy.
- hepatitis C virus
- HCV
- HIV
- co-infection
- daclatasvir
- sofosbuvir
- NS5A
- combination antiretroviral therapy
- cART
- ALLY 2
- NCT02032888
- gastroenterology clinical trials
Kenneth Sherman, MD, PhD, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA, presented the findings of ALLY 2 [NCT02032888], a phase 3, randomized, open-label clinical trial, which demonstrated a sustained virologic response for 12 weeks (SVR12) in 97% of patients coinfected with genotypes 1 to 4 hepatitis C virus (HCV) and HIV. Patients were treated with the NS5A inhibitor daclatasvir (DCV) and NS5B inhibitor sofosbuvir (SOF), which allowed for treatment without having to modify the combination antiretroviral therapy (cART) regimen.
About 130 to 150 million people are infected with chronic HCV worldwide [WHO. http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed June 3, 2015], including an estimated 3 to 4 million in the United States [CDC. http://www.cdc.gov/hepatitis/hcv/statisticshcv.htm. Accessed June 3, 2015]. Up to 20% of people with chronic HCV will develop cirrhosis, with about the same proportion progressing to liver cancer [WHO. http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed June 3, 2015].
HIV/HCV coinfection accelerates progression of HCV liver disease [Macías J et al. Hepatology. 2009] and hinders pegylated interferon treatment [Grint D et al. HIV Med. 2013]. A treatment regimen directed at HIV/HCV co-infected patients must not compromise cART. The positive efficacy and safety results of the DCV + SOF combination in chronic HCV monoinfection [Sulkowski MS et al. N Engl J Med. 2014] prompted ALLY 2, which evaluated the combination treatment in HIV/HCV coinfected patients on a broad range of antiretroviral therapies.
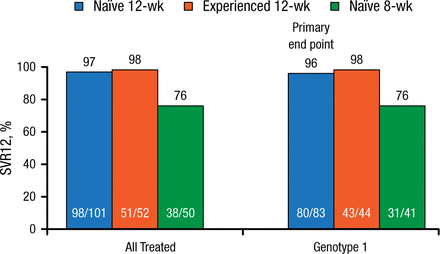
The ALLY 2 trial involved 203 patients who were treatment-naïve for HCV (n = 151) or who had been treated previously (n = 52). The treatment-naïve patients were randomized 2:1 to DCV 30/60/90 mg plus SOF 400 mg QD for either 12 weeks (n = 101) or 8 weeks (n = 50). The treatment-experienced patients received 12 weeks of therapy. The standard DCV dose was 60 mg, with the dose being adjusted according to cART: 30 mg in those receiving ritonavir-boosted protease inhibitors and 90 mg when cART included nonnucleoside reverse transcriptase inhibitors, except for rilpivirine. At baseline, the 3 arms were comparable in demographic, clinical, and prior HIV treatment characteristics. The primary end point, SVR12 in the treatment-naïve patients with HCV genotype 1 treated for 12 weeks, was 96% (Figure 1).
ALLY 2 Results: SVR12 (Intention to Treat) by Treatment Group
SVR12, sustained virologic response for 12 wk.
Reproduced with permission from K Sherman, MD.
Patients with baseline NS5A resistant-associated variants achieved SVR12 at a rate of 96% (22/23) when treated for 12 weeks, and 70% (7/10) when treated for 8 weeks. Treatments did not compromise HIV control, as indicated by the unwavering mean CD4 count and continued HIV virologic suppression throughout the treatment period.
The frequency of serious adverse events was low, occurring in 1.0%, 5.8%, and 0.0% of the naïve/12-week, experienced/12-week, and naïve/8-week arms, respectively. The adverse events commonly comprised fatigue, nausea, headache, and diarrhea. One death occurred after stopping study treatment in the 8-week arm as a result of treatment-unrelated cardiac arrest.
ALLY 2 provides evidence of the efficacy and safety of the DCV + SOF combination in patients coinfected with HIV/HCV, whether or not they had been treated before for HCV. The treatment benefit does not come at the expense of compromised treatment of HIV infection.
- © 2015 SAGE Publications