Summary
In elderly or renally impaired volunteers, the antibody fragment idarucizumab rapidly and completely reversed the anticoagulation effect of dabigatran, sustained for at least 24 hours. Re-administration of dabigatran after 24 hours resulted in anticoagulation similar to that observed during initial treatment. There were no clinically relevant drug-related events; infusion-related effects were similar between arms.
- dabigatran antidote
- anticoagulation
- reversal
- idarucizumab
- dabigatran
- Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of BI 655075 and Establishment of BI 655075 Dose(s) Effective to Reverse Prolongation of Blood Coagulation Time by Dabigatran
- NCT01955720
The dabigatran antidote, idarucizumab, immediately and completely reversed anticoagulation by dabigatran in elderly and renally impaired volunteers, an effect that lasted for at least 24 hours. Joachim Stangier, PhD, Boehringer Ingelheim Pharma GmBH & Co KG, Biberach, Germany, presented data from the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of BI 655075 and Establishment of BI 655075 Dose(s) Effective to Reverse Prolongation of Blood Coagulation Time by Dabigatran study [NCT01955720].
Idarucizumab is the antigen-binding fragment of a humanized antibody that specifically targets dabigatran, a non-warfarin oral anticoagulant. Developed as an antidote to dabigatran, idarucizumab restored coagulation after easy and rapid intravenous administration. Key characteristics of idarucizumab are its initial short half-life of about 45 minutes and terminal half-life of 4.5 to 9 hours and that it is eliminated primarily renally through renal excretion and catabolism. A previous study in heathy male volunteers demonstrated that idarucizumab immediately and completely reversed the anticoagulation effect of dabigatran based on clotting times measured by diluted thrombin time (dTT), ecarin clotting time (ECT), activated partial thromboplastin time (aPTT), and thrombin time (TT) [Glund S et al. Circulation. 2013]. The purpose of the current study was to further evaluate the effect of idarucizumab on anticoagulation reversal in elderly and renally impaired volunteers treated with dabigatran etexilate (DE).
In this double-blind, randomized, 2-way crossover trial, 46 volunteers underwent 2 treatment periods, with a 6-day washout in between. During the first treatment period, patients received DE (220 mg, or 150 mg in the renally impaired) for 3 days, followed by a 5-minute infusion of idarucizumab or placebo about 2 hours after the last dose of dabigatran. A subset group of volunteers were re-treated with dabigatran 24 hours after the idarucizumab infusion. The study protocol was completed by all volunteers. The median peak dabigatran concentration was similar to that typically experienced by patients with atrial fibrillation.
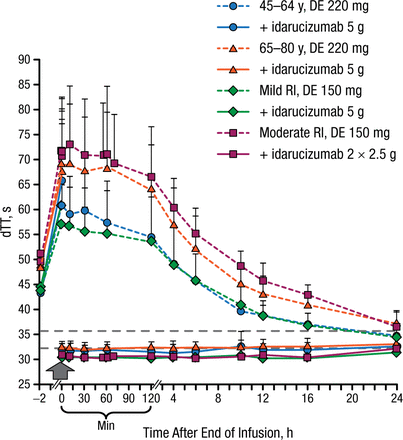
In both age groups (45 to 64 and 65 to 80 years) and in patients with mild or moderate renal impairment, idarucizumab immediately reversed clotting times to baseline levels, as measured by dTT, ECT, aPTT, and TT, which was sustained for at least 24 hours (Figure 1). Anticoagulation was restored to initial levels when dabigatran was readministered 24 hours after the idarucizumab infusion.
Effect of Idarucizumab on the Anticoagulation Effect of Dabigatran
DE, dabigatran etexilate; dTT, diluted thrombin time; RI, renal impairment (CLcr: mild RI ≥ 60 to < 90 mL/min; moderate RI ≥ 30 to < 60 mL/min); TT, thrombin time.
Reproduced with permission from J Stangier, PhD.
There were no clinically relevant drug-related adverse events in the study, and the rates of adverse events and local reactions were similar between the idarucizumab and placebo arms. In patients who received idarucizumab, there was a dose-dependent elevation in urine protein and low-weight proteins that returned to normal values within 24 hours.
Dr Stangier concluded that the data from this and other studies that have evaluated idarucizumab show that it is a specific antidote for anticoagulation caused by dabigatran and that it is well tolerated. In addition, the multicenter, phase 3 RE-VERSE AD trial [NCT02104947] was initiated in May 2014 and will evaluate the efficacy and safety of dabigatran reversal by idarucizumab in patients who are taking dabigatran and present with a major bleeding event or require emergency surgery for other conditions.
- © 2014 SAGE Publications