Summary
The safety and efficacy of oral anticoagulation with warfarin or the nonwarfarin oral anticoagulants have not been well established in settings such as cardioversion or ablation for atrial fibrillation, coronary artery disease, percutaneous coronary intervention, and stent placement. Recent meta-analyses and randomized trials provide new data to consider regarding the positioning of oral anticoagulation in these settings.
- atrial fibrillation
- warfarin
- oral anticoagulants
- non–vitamin K antagonist oral anticoagulants
- NOACs
- vitamin K antagonist
- coronary artery disease
- percutaneous coronary intervention
- CAD
- PCI
- cardiology & cardiovascular medicine guidelines
- myocardial infarction
Questions about the use of oral anticoagulants (OACs)—particularly, the nonwarfarin non–vitamin K antagonist OACs (NOACs)—remain for patients with atrial fibrillation (AF) who require cardioversion, have coronary artery disease (CAD), or require percutaneous coronary intervention (PCI). Greg C. Flaker, MD, University of Missouri Health System, Columbia, Missouri, USA, discussed cardioversion in patients receiving an anticoagulant.
OAC reduces the risk of stroke and systemic embolism (SSE) in patients who undergo cardioversion, with similar rates of SSE among patients who receive a vitamin K antagonist (VKA), a NOAC, or transesophageal echocardiograph–guided cardioversion, as shown in several pivotal NOAC trials (Table 1).
Relative Comparison of Outcomes After Cardioversion in Patients on Anticoagulation
According to Dr Flaker, these data suggest that the use of transesophageal echocardiograph to guide cardioversion does not lower the risk of cardiovascular events and that the risk of cardiovascular events is similar regardless of the type of NOAC used.
Some patients with AF require ablation or device implantation in addition to cardioversion. In these cases, the question is how to manage the NOACs during the periprocedural period. Saverio J. Barbera, MD, Stony Brook Medicine, Stony Brook, New York, USA, stated that one must consider the risks and benefits of either stopping or continuing anticoagulation, as well as individual patient characteristics, the type of procedure, or the procedural technique used. Stopping anticoagulation reduces the risk of pocket hematomas, hemothorax, and cardiac tamponade, but the risk of deep vein thrombosis, pulmonary embolism, and stroke is increased.
The Bruise Control study [Birnie DH et al. N Engl J Med. 2013] evaluated continued warfarin use vs heparin bridging during pacemaker or defibrillator implantation and demonstrated that continued warfarin was favored over bridging among all subgroups, including age, use of antiplatelet therapy, type of device surgery, duration of procedure, and presence of mechanical valve.
In a substudy of the RE-LY trial, periprocedural anticoagulation resulted in similar rates of bleeding between the dabigatran and warfarin arms [Healey JS et al. Circulation. 2012]. In another analysis, patients taking dabigatran who underwent device implantation did not experience any serious bleeding or thromboembolic events [Rowley CP et al. Am J Cardiol. 2013]. In a “real world” study, patients taking dabigatran or rivaroxaban who underwent cardiac rhythm device implantation experienced similar rates of bleeding and thromboembolic events [Kosiuk J et al. Europace. 2014].
For ablation, 2 meta-analyses demonstrated that continued anticoagulation during catheter ablation decreased the risk of stroke or thromboembolism and major bleeding, with additional benefits when rivaroxaban or dabigatran was used instead of warfarin (Table 2).
Periprocedural Anticoagulation During Ablation for Atrial Fibrillation
According to these data, Dr Barbera stated that uninterrupted warfarin is safe and effective during the periprocedural period for device implantation or ablation. However, more prospective studies are needed to determine the safety and efficacy of NOACs in this setting.
The safety and efficacy of anticoagulation in patients who require PCI and dual antiplatelet therapy (DAPT) are also under investigation. Werner Jung, MD, University of Freiburg, Villingen-Schwenningen, Germany, stated that about 30% of patients with AF also have indications for DAPT because of CAD [Markowtiz SM. J Am Coll Cardiol. 2013]. Although OACs are indicated for the reduction of SSE in AF, DAPT has been demonstrated to provide better protection than OACs after stent implantation in patients without AF. This situation raises the question of whether triple therapy (TT)—DAPT plus anticoagulation—is beneficial.
In a meta-analysis of 9 trials, TT was favored for a significant reduction in all-cause mortality (OR, 0.59; 95% CI, 0.39 to 0.90; P = .01), and there was a higher incidence of ischemic stroke in the DAPT arm (OR, 0.38; 95% CI, 0.12 to 1.22; P = .11) [Zhao HJ et al. Chest. 2011]. However, risk of major bleeding was significantly increased over 6 months after implantation (OR, 2.12; 95% CI, 1.05 to 4.29; P = .04). In the WOEST study [Dewilde WJM et al. Lancet. 2013], the primary end point of any bleeding occurred in 44.4% of patients in the TT arm, compared with 19.4% in the double-therapy arm (HR for DAPT vs TT, 0.36; 95% CI, 0.26 to 0.50; P < .0001). The secondary end point of death, myocardial infarction (MI), stroke, target vessel revascularization, and stent thrombosis was also greater in the TT arm (17.6% vs 11.1% with DAPT; HR for DAPT vs TT, 0.60; 95% CI, 0.38 to 0.94; P = .025).
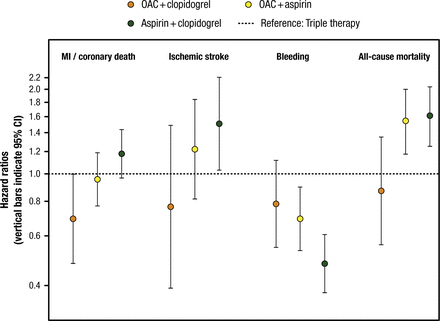
However, an OAC plus 1 antiplatelet agent appears to provide benefit without an increased risk of bleeding. In an analysis of the Danish National Patient Registry, an OAC plus clopidogrel reduced the risk of MI or coronary death, ischemic stroke, bleeding, and all-cause mortality compared with TT (Figure 1) [Lamberts M et al. J Am Coll Cardiol. 2013]. Similarly, an analysis of the AFCAS Registry found that the safety and efficacy of VKA plus clopidogrel was comparable with DAPT or VKA plus DAPT [Rubbioli A et al. Clin Cardiol. 2014]. A meta-analysis of 6 randomized controlled trials found that with OAC plus clopidogrel, there was a significant reduction in bleeding (OR, 0.79; 95% CI, 0.64 to 0.98) without affecting the composite of death, MI, stroke, and stent thrombosis (OR, 0.90; 95% CI, 0.69 to 1.23) compared with TT [D’Ascenzo F et al. Am J Cardiol. 2015].
Effect of OAC Plus Antiplatelet Therapy After Percutaneous Coronary Intervention
Triple therapy (oral anticoagulant [OAC] plus aspirin plus clopidogrel [dotted line]) is used as a reference (hazard ratio = 1.00). Orange circles indicate OAC plus clopidogrel; yellow circles indicate OAC plus aspirin; green circles indicate aspirin plus clopidogrel. MI = myocardial infarction.
Reprinted from J Am Coll Cardiol, Vol. 62, Lamberts M et al, Oral Anticoagulation and Antiplatelets in Atrial Fibrillation Patients After Myocardial Infarction and Coronary Intervention, Pages No. 981-989, Copyright (2013), with permission from American College of Cardiology Foundation.
The ISAR-TRIPLE trial [Fiedler KA et al. J Am Coll Cardiol. 2015] evaluated shortening the duration of antiplatelet therapy to 6 weeks after stent implantation in patients receiving OACs. There was no difference between the 6-week and 6-month groups in the primary end point, which was a composite of death, MI, definite stent thrombosis, stroke, or TIMI major bleeding at 9 months. For the secondary end point of BARC bleeding ≥ 2, there was no difference between the groups at 9 months (18.4% vs 21.3%; P = .41); however, a post hoc analysis from 6 weeks to 9 months showed a trend toward a difference between the groups based on the per-protocol analysis at 9 months (7.6% vs 12.2%; P = .07).
Prof Jung stated that, based on the current data, a VKA plus clopidogrel appears to be a reasonable option to TT in selected patients who require a stent.
Risk stratification for stroke and bleeding in patients with AF in clinical practice was discussed by Gregory Y. H. Lip, MD, University of Birmingham, Birmingham, United Kingdom. The CHA2DS2-VASc score was adopted by the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society as a guideline for the management of patients with AF for stroke risk assessment [January CT et al. Circulation. 2014]. The CHA2DS2-VASc score was found to have greater sensitivity for identifying truly low-risk patients with AF who do not need OACs [Potpara TS et al. Circ Arrhythmia Electrophysiol. 2012].
In AF, low risk is defined as no additional risk factors, which is a CHA2DS2-VASc score of 0 in men and 1 in women [Lip GY et al. J Am Coll Cardiol. 2015]. Even 1 additional risk factor increases the risk ischemic stroke or death; however, warfarin reduced the risk without substantially increasing risk of intracranial hemorrhage.
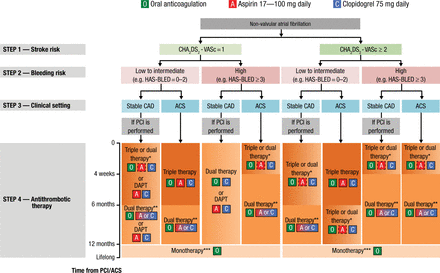
HAS-BLED has been demonstrated to perform better than other bleeding risk scores [Lip GY et al. Circ Arrhythm Electrophysiol. 2012] and the CHA2DS2-VASc or CHADS2 scores [Apostolakis S et al. Thromb Haemost. 2013]. For everyday clinical practice, Prof Lip recommended using the CHA2DS2-VASc to identify patients who do not need OACs and the HAS-BLED score to guide therapy selection and identify reversible risk factors. He pointed out that OACs should not be withheld due to a high HAS-BLED score. The 2014 European consensus document on management of antithrombotic therapy in AF patients provides an algorithm for determining the optimal treatment of patients with AF and CAD or ACS (Figure 2) [Lip GY et al. Eur Heart J. 2014].
Algorithm for Treatment Selection
Choice of antithrombotic therapy, including combination strategies of oral anticoagulation (O), aspirin (A) and/or clopidogrel (C). For Step 4, background colour and gradients reflect the intensity of antithrombotic therapy (i.e. dark background colour = high intensity; light background colour = low intensity). Solid boxes represent recommended drugs. Dashed boxes represent optional drugs depending on clinical judgement. New generation drug-eluting stent is generally preferable over bare-metal stent, particularly in patients at low bleeding risk (HAS-BLED 0–2). When vitamin K antagonists are used as part of triple therapy, international normalized ratio should be targeted at 2.0–2.5 and the time in the therapeutic range should be >70%.
ACS, acute coronary syndromes; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; PCI, percutaneous coronary intervention.
*Dual therapy with oral anticoagulation and clopidogrel may be considered in selected patients.
**Aspirin as an alternative to clopidogrel may be considered in patients on dual therapy (i.e. oral anticoagulation plus single antiplatelet).
***Dual therapy with oral anticoagulation and an antiplatelet agent (aspirin or clopidogrel) may be considered in patients at very high risk of coronary events.
Reprinted from Lip GYH et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J. 2014; 35 (45): 3155-3179. By permission of European Society of Cardiology.
In conclusion, OACs may be safe and effective during cardioversion and during the periprocedural period for device implantation and ablation. For patients who require PCI, an OAC plus 1 antiplatelet agent, such as clopidogrel, may be beneficial over TT.
- © 2015 SAGE Publications