Summary
This article presents data from three clinical trials, and also discussed off-label use of argatroban for ischemic stroke. The results demonstrated positive clinical outcomes, indicating that argatroban appears to be safe and promising, both as monotherapy and in combination with intravenous tissue plasminogen activator (t-PA) for patients with ischemic stroke.
- Ischemia
- Cerebrovascular Disease
- Neurology
- Ischemia
- Cerebrovascular Disease
Andrew D. Barreto, MD, University of Texas Health Science Center, Houston, Texas, USA, presented data from three clinical trials, and also discussed off-label use of argatroban for ischemic stroke. The results demonstrated positive clinical outcomes, indicating that argatroban appears to be safe and promising, both as monotherapy and in combination with intravenous tissue plasminogen activator (t-PA) for patients with ischemic stroke
In the United States, argatroban is currently only approved by the Food and Drug Administration for anticoagulation in patients with heparin-induced thrombocytopenia (HIT), and for percutaneous coronary intervention in those with or at-risk for HIT. However, it is approved for use in Japan in the setting of atherosclerotic ischemic stroke.
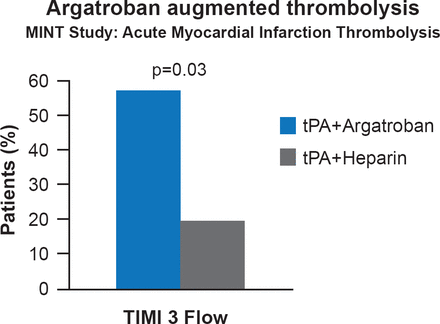
Argatroban is a small molecule, synthetic, thrombin inhibitor that directly and reversibly blocks the active site of thrombin. It is highly selective for thrombin and blocks all of its physiological effects at clinically useful doses. In patients with acute myocardial infarction, argatroban has been found to augment thrombolysis (Figure 1), and seems to be more beneficial than heparin by improving microcirculatory blood flow and enhancing reperfusion of large occlusions [Jang IK et al. J Am Coll Cardiol 1999].
Argatroban Enhances Thrombolysis
MINT=myocardial infarction with novastan and t-PA; t-PA=tissue plasminogen activator; TIMI 3= thrombolysis in myocardial infarction grade 3 flow.
Reproduced from Jang IK et al. A multicenter, randomized study of argatroban versus heparin as adjunct to tissue plasminogen activator (t-PA) in acute myocardial infarction: myocardial infarction with novastan and t-PA (MINT) study. J Am Coll Cardiol 1997; 33(7):1879–1885. With permission from Elsevier.
Three trials have now been published (Japanese Study, ARGIS, ARTSS-1), and a fourth is ongoing (ARTSS2), reporting data that suggest that argatroban may also be beneficial in patients with stroke.
Data from a Japanese, multicenter, Phase 2, randomized, double-blind, placebo-controlled study were published in 1997 [Kobayashi S, Tazaki Y. Semin Thromb Hemost]. Patients (n=119) were treated either with argatroban (60 mg/day for 48 hours, then 10 mg BID for 5 days; n=60) or placebo (n=59). Exclusion criteria included lacunar strokes and suspected cerebral embolism, and the primary endpoint was global improvement of clinical symptoms at 28 days (identifying patients in both groups who were “markedly improved” or “improved”). Overall, data demonstrated significant improvements in the treatment arm compared with placebo (54.2% vs 23.7%; p<0.01). Only one patient (2%) in the treatment arm experienced a complication during the study. This was reported as a mild hemorrhagic cerebral infarction, with similar complications reported in two participants in the placebo arm. The study had some limitations, including a lack of baseline NIHSS or long-term follow-up data, but nevertheless, the results did indicate that argatroban was safe and effective for treatment of patients with acute cerebral thrombosis.
The Argatroban Anticoagulation in Patients With Acute Ischemic Stroke [ARGIS-1] study was a North American, multicenter, Phase 2, randomized, double-blind, placebo-controlled study [LaMonte MP et al. Stroke 2004]. Non-t-PA-treated patients (n=171) with acute stroke <12 hours and NIHSS scores of 5 to 22 were randomized to 3 study arms for 5 days: low dose (continuous intravenous infusion of argatroban: 100 μg/kg bolus, then 1 μg/kg/min; partial thromboplastin time [PTT] 1.75x baseline; n=59); high dose (continuous intravenous infusion of argatroban: 100 μg/kg bolus, then 3 μg/kg/min; PTT 2.25x baseline; n=58); placebo (n=54). The primary endpoint was symptomatic intracranial hemorrhage (ICH) at 30 days. The mean time from onset of symptoms to treatment was almost 9 hours, and the median NIHSS was 9. Five cases of symptomatic ICH were reported, two of which occurred more than 7 days after argatroban treatment ended (overall ICH rates in the high dose, low dose, and placebo arms were 5.1%, 3.4%, and 0%, respectively; p≥0.18). There was no significant difference between the groups in asymptomatic ICH, major systemic hemorrhage, or 90-day mortality. The percentage of participants who reached mRS score of 0 to 2 was 51, 45, and 54 in the high-dose, low-dose, and placebo groups, respectively.
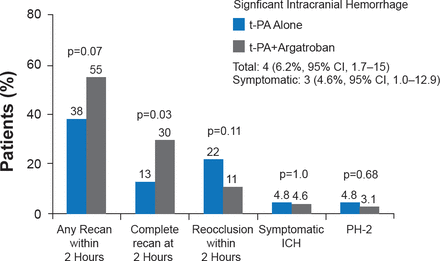
The Argatroban t-PA Stroke Study [ARTSS-1] was an open-label, single-arm, pilot Phase 2a safety study to investigate t-PA plus argatroban in patients with ischemic stroke due to proximal intracranial occlusion [Barreto AD et al. Stroke 2012]. Inclusion criteria included patients aged 18 to 85 years; patients receiving t-PA within 4.5 hours of symptom onset; clot causing partial or complete occlusion of proximal large artery by transcranial Doppler imaging (TCD) or computed tomography-angiogram (CTA); and INR ≤1.5 and no known liver disease. Participants (n=65; mean age 63±14 years; median NIHSS=13) were initiated on a low-dose argatroban treatment regimen (100 μg/kg bolus, then 1 μg/kg/min; PTT 1.75x baseline for 48 hours). The aim was to administer the argatroban bolus during the t-PA infusion, with as much treatment overlap as possible. The primary outcome was the incidence of significant ICH or parenchymal hemorrhage type 2 (PH-2). Secondary outcomes were rate of ultra-early (2 hours) recanalization and Day 7 mRS. At baseline, the median time from symptom onset to t-PA bolus administration was 128 minutes, and the median overlap time for t-PA infusion plus argatroban infusion was 17 minutes; target anticoagulation was attained at a median of 3 hours. Significant ICH was reported in four patients (6.2%; 95% CI, 1.7 to 15.0), three of whom were symptomatic (4.6%; 95% CI, 0.9 to 12.9; Figure 2). Seven participants died within the first 7 days of the study.
Incidence of ICH, Recanalization, and Reocclusion in ARTSS-1
ICH=intracranial hemorrhage; PH-2=parenchymal hemorrhage type-2; t-PA=tissue plasminogen activator. tPA-alone historical controls were prospectively planned and obtained from the CLOTBUST study [Alexandrov AV et al. N Engl J Med 2004].
Reproduced with permission from AD Barreto, MD.
A matched case-control analysis was subsequently performed. This aimed to assess the safety and efficacy of argatroban plus t-PA in patients in the ARTSS-1 trial, compared with a matched group of patients from the National Institute of Neurological Disorders and Stroke [NINDS] t-PA stroke study who were treated with t-PA alone [NINDS. N Engl J Med 1995]. Analysis revealed no signal of increased mortality or symptomatic hemorrhage when argatroban was added to the treatment regimen, and there was a non-significant trend toward improved excellent functional outcomes at 90 days (14% relative improvement of 0–1 on the mRS) in patients who also received argatroban in the ARTSS-1 trial [Barreto AD et al. Stroke 2014].
Although these data are reassuring, Dr. Barreto noted that concurrent controls in a randomized clinical trial are now necessary. And to this end, ARTSS-2, a Phase 2b, randomized, international, multi-center study is currently recruiting participants [NCT01464788]. This is an open-label, blinded imaging and 90-day outcome assessments trial to evaluate overall treatment benefit among stroke patients of any age (with no NIHSS limitations) treated with t-PA who are randomized to one of three arms (n=105): low-dose argatroban, high-dose argatroban, or neither. The study is planned to complete in early 2015 and will examine the following:
-
▪ Early recanalization using TCD or CTA at 2 to 3 hours post t-PA administration
-
▪ Efficacy as measured by 90-day mRS 0–1 utilizing a Bayesian analytical approach
-
▪ Incremental quality adjusted life years gained via a cost-utility analysis
Dr. Barreto concluded that argatroban appears to be safe, both as monotherapy and in combination with intravenous t-PA for patients with ischemic stroke. However, he added that ongoing studies are essential to investigate whether it is safe to combine both drugs in a high-dose regimen, to compare early recanalization rates with concurrent controls, and to determine the efficacy signal to allow this therapy to move forward clinically.
- © 2014 MD Conference Express®