Summary
Strategies to reduce delays to treatment and shorten the time to recanalization are required to improve outcomes with endovascular therapy (ET). Although procedural times are reduced with new technologies, the focus must remain on the front end of care, which consumes 75% of the time to recanalization. This article discusses MRI combined with CT for improved patient selection and outcomes, as well as lessons from the ongoing Endovascular Treatment for Small Core and Proximal Occlusion Ischemic study [ESCAPE; NCT01778335].
- Neuroimaging
- Interventional Techniques & Devices
- Cerebrovascular Disease
- Neurology
- Neuroimaging
- Interventional Techniques & Devices
- Cerebrovascular Disease
Strategies to reduce delays to treatment and shorten the time to recanalization are required to improve outcomes with endovascular therapy (ET). Although procedural times are reduced with new technologies, the focus must remain on the front end of care, which consumes 75% of the time to recanalization, said Rishi Gupta, MD, Wellstar Kennestone Hospital, Marietta, Georgia, USA.
Transferring stroke patients to a comprehensive stroke center (CSC) costs time. The median transfer time was 104 minutes for short distance transfers of 14.7 miles in a study of 132 patients at Rush Medical Center in Chicago, Illinois, and consumed 30% of the time in the patient care pathway [Prabhakaran S et al. Stroke 2011]. A rapid decrement in the probability of intra-arterial treatment (IAT) of 3% per minute was found after 46 minutes had elapsed.
The time from computed tomography (CT) imaging to groin puncture (picture-to-puncture; P2P) is a positive predictive variable for outcomes. A single-center, retrospective study of P2P comparing patients who were transferred or presented at their local emergency department (ED) provided “eye-opening” messages said Dr. Gupta [Sun CH et al. Circulation 2013]. Every 90-minute increase in the time to treatment resulted in fewer patients having a good outcome (55.8% for <90 minutes vs 32.9% for 91 to 180 minutes). Only 29% of the transferred patients achieved an mRS score of 0 to 2, compared with 51% of patients with local ED admission because of the longer door-to-puncture (D2P) times of 300 versus 177 minutes.
Two strategies to reduce treatment delay are physician transfer to the non-CSC and improving prehospital triage. A study in Shanghai, China, showed transferring the physician, compared with transferring the patient, reduced door-to-balloon time by nearly 50 minutes (92 vs 141 minutes) and increased the proportion of patients treated in <90 minutes (36% vs 13%, respectively) [Zhang Q et al. Circ Cardiovasc Qual Outcomes 2011].
Efforts to improve prehospital triage include using the Los Angeles Motor Scale (LAMS) to quantify the degree of weakness, which has an 85% accuracy for detecting large vessel occlusion [Nazliel B et al. Stroke 2008].
The protocol at Wellstar Kennestone Hospital includes using the LAMS and instructing emergency medical services (EMS) to take patients with symptoms of stroke plus hemiplegia directly to the CSC if travel time is <30 minutes.
MRI COMBINED WITH CT IMPROVED PATIENT SELECTION AND OUTCOMES
The value of noncontrast CT to rapidly rule out hemorrhage in patients presenting with a stroke so intravenous tissue plasminogen activator (t-PA) can be administered is accepted. Other imaging strategies are being investigated to improve patient selection for acute ET, including CT angiography (CTA) to determine large vessel occlusion and evaluate collaterals, CT perfusion, and magnetic resonance imaging (MRI). However, there are conflicting data on their benefit and currently there is no accepted standard for advanced imaging.
The Cleveland Clinic converted from a CT-based to an MRI-based imaging protocol in April 2010 to improve decision-making in patients presenting with a stroke. The hyperacute (HA-MRI) protocol, added to CT and CTA, resulted from intensive planning, testing and quality control, even determining the fastest path through the hospital and elevators to ensure rapid completion, said Muhammad Shazam Hussain, MD, Cleveland Clinic, Cleveland, Ohio, USA.
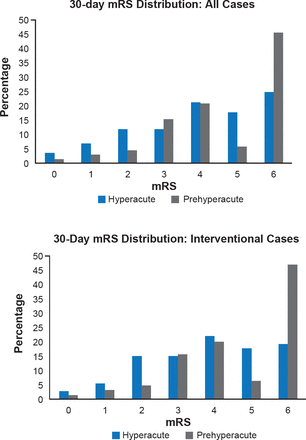
The addition of pretreatment MRI to CT and CTA, compared with CT and CTA alone, improved 30- and 90-day mRS scores in patients with large vessel occlusion (Figure 1), according to results of their retrospective study to evaluate the benefit of the new protocol [Wisco D et al. Stroke 2014].
mRS Score Outcomes by Imaging Protocol
Hyperacute=pretreatment magnetic resonance imaging protocol; mRS=modified Rankin Scale score; prehyperacute=computed tomography and computed tomography angiography protocol.
Reproduced from Wisco D et al. Addition of hyperacute MRI AIDS in patient selection, decreasing the use of endovascular stroke therapy. Stroke 2014;45(2):467–472. With permission from Lippincott Williams and Wilkins.
Of 179 HA-MRI patients, 34.1% had atrial fibrillation at baseline, compared with 48.7% of the 88 prehyperacute patients. The patients were 70 years old, 56% were women, and the NIHSS was 15.8 to 16.4 at baseline.
No significant difference was found in the time to ET with HA-MRI (390 vs 407 minutes prehyperacute), suggesting it was possible to insert HA-MRI without significant time delay, said Dr. Hussain.
The proportion of patients who had an ET was reduced to 51.7% with HA-MRI from 96.6% (p<0.01), mainly because large infarctions not seen on CT were seen on MRI. In the HA-MRI group, compared with the prehyperacute group, more patients achieved a mRS score of 0 to 2 at 30 days (23.6% vs 9.1%; p=0.01) and fewer died (33.1% vs 45.5%; p=0.09).
The probability of clinical outcomes in all patients and in those who had ET is shown in Table 1. Age, baseline NIHSS, and pretreatment MRI were significantly associated with improved outcomes on multivariable analysis (p<0.05). The daily direct costs were reduced by 24.5% with HA-MRI compared with CT and CTA (95% CI, 14.1% to 33.7%; p<0.0001).
Probability of Clinical Outcomes in Cleveland Clinic Study With Pretreatment MRI
LESSONS FROM ESCAPE: TEAM WORK AND PROTOCOLS IMPROVING TIME TO RECANALIZATION
Preliminary data from the ongoing Endovascular Treatment for Small Core and Proximal Occlusion Ischemic study [ESCAPE; NCT01778335] suggest that an imaging-to-recanalization time of <90 minutes was achieved in ∼80% of endovascular patients, who received thrombectomy or thrombolysis. Mayank Goyal, MD, University of Calgary Calgary, Alberta, Canada, said the study is examining whether or not a patient with an acute ischemic stroke should go to the catheterization laboratory, not whether ET is superior to t-PA.
The key to achieving these results is having two teams parallel process patient care. The stroke team is responsible for clinical items, including trial checklist, blood work, informed consent, moving the patient, and assessing need for anesthesia. Simultaneously, based on data only from the CTA, the interventionalist plans and prepares for the procedure. Notably, Prof. Goyal stressed the need for Bayesian thinking, that is, not relying on a singular piece of information, but putting together all key pieces of available information for decision-making. This is a particular focus for training to further improve the proportion of patients achieving the <90-minute benchmark.
At his institution, Prof. Goyal is informed as soon as a patient is randomized to ET and he is scrubbed with needle in hand when the patient arrives. To prevent wastage, equipment is standardized in case the patient is randomized to a conservative approach, and expensive items are not opened until time of use. He recommends the time immediately after the procedure be used to clean and organize and plan what to do next if the vessel is not open. Using parallel processing, his institution achieved in one example case times to recanalization from the door of 50 minutes, from CT 42 minutes, from clot recognition 5 minutes, and from puncture 8 minutes.
Clear plans must be established for the prehospital and ED phases, as well as for each type of procedure. Clear protocols for angiography must be established to receive stroke patients 24/7, including preparing in advance stroke trays with a basic set of items and training staff.
A critical issue that produces delays is obtaining informed consent, which took an average of 30 minutes in the IMS-III trial, and affects only the endovascular arm of the trial, Prof. Goyal stated. To obtain efficient and fast consent, he recommends training staff and preparing an institutional response to the main questions asked at the time of informed consent.
- © 2014 MD Conference Express®