Summary
This article discusses the use of a novel probe in the positron emission tomography (PET) molecular imaging of thrombosis and thrombolysis in vivo. Diseases including stroke, coronary artery disease, pulmonary embolism, and deep vein thrombosis are often triggered by the formation of a thrombus. Noninvasive assessment of thrombosis is crucial in diagnoses and to monitor disease progression.
- Imaging Modalities
- Neurology Clinical Trials
- Cardiac Imaging Techniques
- Interventional Radiology
- Thrombotic Disorders
- Neuroimaging
- Molecular Imaging
- Ischemia
- Imaging Modalities
- Neurology Clinical Trials
- Neurology
- Cardiac Imaging Techniques
- Interventional Radiology
- Thrombotic Disorders
- Neuroimaging
- Molecular Imaging
- Ischemia
Francesco Blasi, PharmD, PhD, Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA, described the use of a novel probe in the positron emission tomography (PET) molecular imaging of thrombosis and thrombolysis in vivo.
Diseases including stroke, coronary artery disease, pulmonary embolism, and deep vein thrombosis are often triggered by the formation of a thrombus. Noninvasive assessment of thrombosis is crucial in diagnoses and to monitor disease progression. In seeking to develop a thrombus-specific imaging tool, Dr. Blasi and colleagues exploited fibrin as a target (Table 1).
Fibrin Targeting for Thrombus Imaging
In this study, a fibrin-specific probe labeled with 64Cu was used. Fibrin-binding probe 7 (FBP7) has high affinity for fibrin, is metabolically stable, has a short half-life of 18 minutes (rapid clearance from blood) and a favorable biodistribution. The in vivo prowess of FBP7 was evaluated using rat models of crush-induced mural thrombosis and occlusive embolic stroke. PET and computed tomography (CT) imaging were performed following intravenous administration of FBP7. The stroke model also included observations following treatment with recombinant tissue plasminogen activator (rt-PA) to quantitatively assess clot bursting. Following sacrifice, ex-vivo assessments of thrombolysis, and probe biodistribution were also performed.
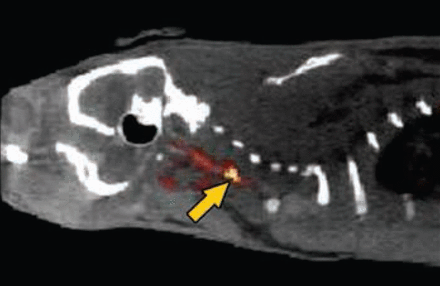
Imaging revealed the specificity of FBP7 for the thrombus (Figure 1). Binding to the thrombus exceeded binding in the left carotid, brain, and heart by ∼4-, 12-, and 2.5-fold, respectively, soon after the injection of FBP7. The binding specificity to the thrombus was maintained with time (90 minutes), while binding to the other regions diminished. Examination at 4 hours post injection revealed the same uptake of FBP7 to the thrombus, but reduced binding in the other regions, resulting in lower background; thrombus binding of the probe exceeded binding to the left carotid, brain, and heart by ∼15-, 65-, and 10-fold, respectively.
FBP7 Detects Mural Thrombosis at the Level of the Common Carotid Artery (arrow). Fused PET-CT image.
Reproduced with permission from F Blasi, PharmD, PhD.
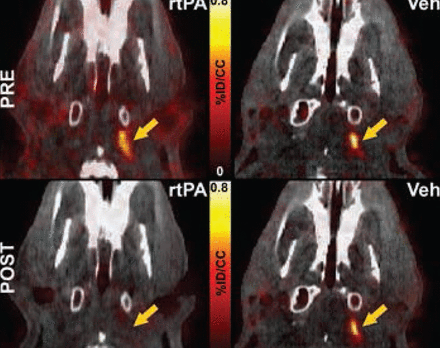
PET-CT imaging in the embolic stroke model revealed the specificity of FBP7 for the detection of the embolus at the level of the internal carotid artery and the middle cerebral artery, common sites of occlusion in human strokes. Furthermore, the dissolution of the embolus following administration of rt-PA was evident, while probe binding remained constant in rats who received vehicle instead of rt-PA (Figure 2).
FBP7 Detects Thrombolysis in the Internal Carotid Artery After Treatment With rt-PA, but Not Vehicle. Fused PET-CT images.
rt-PA=recombinant tissue plasminogen activator; Veh=vehicle.
Reproduced with permission from F Blasi, PharmD, PhD.
Table 2 summarizes the study conclusions.
Study Conclusions
FBP7 is a very promising candidate for clinical testing. Dr. Blasi added that this proof-of-concept study is now being followed by assessments of the capability of the probe to quantify the level of fibrin in thrombi and to detect different stages of the thrombus evolution.
Dr. Blasi received the Mordecai Y. T. Globus New Investigator Award in Stroke at the International Stroke Conference 2014 for his work on molecular imaging of thrombosis.
- © 2014 MD Conference Express®