Summary
Osteonecrosis of the femoral head (ONFH) is a multifactorial and heterogeneous group of disorders that leads to death of the femoral head. This session was devoted to an overview of treatment options for this debilitating disease. This article discusses issues related to etiology, pharmacological management, epigenetics, and surgical approaches to the treatment of osteonecrosis.
- Bone Density & Structure Disorders
- Hip & Knee Conditions
- Orthopaedics
- Bone Density & Structure Disorders
- Hip & Knee Conditions
Osteonecrosis of the femoral head (ONFH) is a multifactorial and heterogeneous group of disorders that leads to death of the femoral head (Figure 1). This session was devoted to an overview of treatment options for this debilitating disease. The speakers discussed issues related to etiology, pharmacological management, epigenetics, and surgical approaches to the treatment of osteonecrosis.
Etiology of Osteonecrosis of the Femoral Head
Reproduced with permission from CJ Glueck, MD.
The natural history of untreated asymptomatic ONFH is poor. A recent literature review [Mont MA et al. J Bone Joint Surg Am 2010] revealed that 59% of these patients progress to symptomatic disease or collapse. Small, medially located lesions had the best prognosis, while patients with sickle cell disease had the highest frequency of progression. Symptoms generally occur after ∼39 months depending on the size of the lesion, location, and stage of the disease.
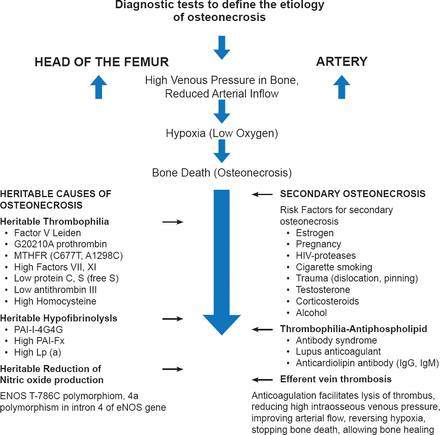
Charles J. Glueck, MD, Jewish Hospital-Mercy Hospitals, Cincinnati, Ohio, USA, discussed the role of clotting system abnormalities in the development of osteonecrosis. The etiology pinpoints venous occlusion as the primary event. Osseous outflow obstruction caused by thrombophilia-hypofibrinolysis-induced venous thrombosis leads to increased intraosseous venous pressure, reduced arterial flow, ischemia, and bone death. If started at Ficat Stage I or II, anticoagulation may improve arterial flow, reverse the hypoxia, stop bone death, relieve pain, and allow bone healing, and thus change the natural history of idiopathic osteonecrosis.
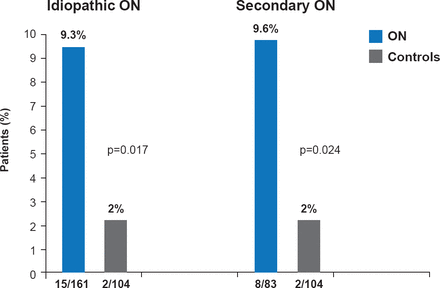
Important measurements include polymerase chain reaction assays to identify the factor V Leiden mutation, which is the most common herita ble mutation associated with osteonecrosis (Figure 2). Serologic tests for high levels of homocysteine, Factors VIII and XI, and resistance to activated protein C, all of which have been implicated in the occurrence of thrombosis and as risk factors for osteonecrosis, should also be performed.
Factor V Leiden Heterozygosity Idiopathic and Secondary Osteonecrosis
ON=osteonecrosis.
Reproduced with permission from CJ Glueck, MD.
Nitric oxide plays an important role in bone angiogenesis, thrombosis, and turnover, all of which are associated with the pathogenesis of osteonecrosis. The T786C mutation in the endothelial nitric oxide synthase gene, which reduces the formation of nitric oxide from L-arginine, is associated with idiopathic osteonecrosis. Oral therapy with L-arginine (9.2 g/day) appears to increase nitric oxide production in patients with T786C homo- or heterozygosity, and may ameliorate osteonecrosis.
In a prospective study, enoxaparin (60 mg/day) administered for 3 months produced a 4-year survival of 80% of hips and decreased the incidence of total hip replacement (THR) compared with untreated controls in patients with heritable thrombophilia-hypofibrinolysis [Glueck CJ et al. Clin Orthrop Relat Res 2005]. In 6 patients (8 hips) heterozygous for the factor V Leiden mutation with Ficat Stage I or II osteonecrosis, there was no progression to Ficat Stage III up to 16 years with initial enoxaparin treatment followed by lifelong full-dose anticoagulation (Coumadin, dabigatran etexilate mesylate, or rivaroxaban). Similar benefit was seen in patients with idiopathic knee osteonecrosis [Glueck CJ et al. Osteonecrosis 2014. In press].
Dr. Glueck recommends long-term (4 to 16 years) use of anticoagulation in patients with idiopathic osteonecrosis (Ficat I-II) who have factor V Leiden heterozygosity or have resistance to activated protein C, as it can stop the progression of osteonecrosis, ameliorate symptoms, and appears to change the natural history of the disease.
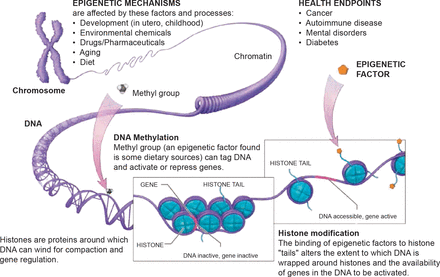
The current definition of epigenetics states that an epigenetic trait is a stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence. The most important part of this definition is the concept of expression of a trait without changes in the DNA sequence. As an example, Javad Parvizi, MD, Rothman Institute, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, USA, noted that children of mothers born during a drought inherit more recessive traits, while those born during a bounty inherit fewer recessive traits. Thus, food (environment), in this instance, silenced bad genes. The two main components of the epigenetic code that control surrounding proteins and act as silencing mechanisms are DNA methylation [Reynard LN et al. Hum Mol Genet 2011] and histone modification, both derived from our food and environment.
There is an inheritance or genetic basis for just about every human ailment, including avascular necrosis (AVN). A 2004 study [Chen WM et al. Am J Hum Genet 2004] mapped the candidate gene for autosomal dominant ANFH to a 15-cM region between D12S1663 and D12S1632 on chromosome 12q13. This was followed by additional studies showing that patients with familial AVN [Liu YF et al. N Engl J Med 2005] and Legg-Calvé-Perthes disease [Su P et al. Arthritis Rheum 2008] carried a mutation in the type II collagen gene (COL2A1). The effects of epigenetics have also been studied in osteosarcoma, osteoarthritis, chrondrogenesis, and rheumatoid disease. Dr. Parvizi believes the pathophysiology of AVN is affected by both the environment (epigenetics) and hereditary factors (genetic predisposition) and the “future is truly bright for epigenetic therapy” (Figure 3).
DNA Alteration
Source: National Institutes of Health Common Fund, Epigenomics.
Michael A. Mont, MD, Sinai Hospital of Baltimore, Baltimore, Maryland, USA, discussed his approach to the management of patients with ONFH. His philosophy centers on using joint preserving procedures for those with early disease, with the objective of forestalling or preventing femoral head collapse and preserving joint function. Key in determining the most appropriate approach for each patient are stage (pre- or post-collapse), lesion size, amount of head depression, and acetabulum involvement.
For nonvascularized bone grafting, Dr. Mont creates a 1.5- to 2-cm trapdoor at the femoral head junction. After removing the necrotic tissue, the lesion created by the trapdoor can be filled with graft material along with the patient's marrow, stem cells, growth factor, as preferred by the surgeon. The trapdoor is then replaced and held in place by pins. The success rate for the trapdoor approach is ∼80% [Seyler TM et al. Clin Orthrop Relat Res 2008; Mont MA et al. Clin Orthrop Relat Res 2003].
Traditional core decompression involves the use of an 8- to 12-mm cannula to remove a core of bone from the femoral head. Complications can occur in ∼10% of patients and additional surgery is needed in ∼30% [Marker DR et al. Clin Orthrop Relat Res 2008]. Dr. Mont uses a percutaneous approach that employs small drill bits (3.2 to 2.4 mm) to make 2 to 3 penetrations (depending on lesion size) into the femoral head under fluoroscopy. The procedure takes 5 to 10 minutes and the wound can be closed with a band aid. Drilling is simpler and safer compared with core decompression. Dr. Mont and colleagues reported an 80% success rate at 2 years in patients with Ficat and Arlet Stage I or II osteonecrosis [Mont MA et al. Clin Orthrop Relat Res 2004]. Post operatively, patients are instructed to reduce hip weight bearing by 50% for 4 to 6 weeks. High-impact activities are not permitted for the first 12 months.
The subcutaneous approach using multiple drilling is a low morbidity minimally invasive procedure, with minimal to no complications. An advantage is that the anterior portions of the femoral head can be reached with drilling that is not possible with conventional core decompression. If drilling fails, bone-grafting procedures can be attempted next.
Philippe Hernigou, Hospital Henri Mondor, University of Paris, Paris, France, discussed the treatment of ONFH with bone marrow replacement procedures.
The first time a patient was treated with stem cells and autologous concentrated bone marrow was in Paris in 1989. Since then Prof. Hernigou has used this technique on >2000 hips with few complications and no fractures when using a small diameter (4 mm) trocar. There was no increase in the incidence of cancer [Hernigou P et al. J Bone Joint Surg Am 2013]. Stem cells help restore blood flow and regenerate blood vessels. Mesenchymal stem cells (MSC) from adult bone marrow are multipotent and can differentiate into fibroblastic, osteogenic, myogenic, adipogenic and reticular cells [Srouji S, Livine E. Mech Ageing Dev 2005]. Among patients with osteonecrosis, the number of MSCs in the femoral head is significantly decreased, making bone repair difficult [Hernigou P et al. J Bone Joint Surg Br 1999]. When treating with MSCs we should strive to load to about 36,000 MSCs (the number present in a healthy 50 cm3 femoral head) [Homma Y et al. Int Orthop 2013]. The technique involves aspiration of red marrow from the iliac crest, concentration of bone marrow with centrifugation to obtain mononuclear cells with MSCs, and then reinjection of concentrate with 3 mm trephine through the great trochanter.
Radiological and anatomical evaluation of patients with Stage I and II osteonecrosis treated with core decompression and concentrated bone marrow show better outcomes (fewer collapses, more revascularization) than those who are treated with core decompression alone. Patients receiving a greater number of progenitor cells also had better outcomes [Hernigou P, Beaujean F. Clin Orthop Relat Res 2002]. Even patients with more advanced disease (Steinberg Stage III) can have successful outcomes with this procedure [Hernigou P et al. Indian J Orthop 2009].
Other techniques under study include expanding the number of autologous cells by culture with and without scaffold and growth factors, allogeneic stem cell grafts, and gene transfer. New methods of administration include directly into the bones, intra-arterial circumflex injection, and intravenously for multifocal osteonecrosis. Dr. Hernigou concluded that autologous concentrate bone marrow administration is safe and effective with few complications. Allogeneic stem cell production and the expansion approach to producing autologous cells by culture are promising new approaches if new studies can address the safety and cost issues.
The goals of treatment in patients with osteonecrosis are to relieve pain, prevent collapse, and delay THR. Jay R. Lieberman, MD, University of Southern California, Los Angeles, California, USA, provided some tips for femoral head preservation and reviewed a few of the new bone graft substitutes. Careful patient selection is key. Patients with fairly normal range of motion of the hip joint have the best prognosis for preservation, while those with loss of internal rotation, femoral head collapse, or a crescent sign due to bone delamination are poor candidates. Edema on MRI should be considered a sign for caution. Dr. Lieberman recommends the University of Pennsylvania Staging System (Table 1).
University of Pennsylvania Staging System
Nonoperative options include anticoagulants (enoxaparin), bisphosphonates, and biophysical modalities. Operative options are core decompression with or without grafting, osteotomy, and arthroplasty. All can be useful once lesion size and location, amount of weight-bearing surface on the femoral and staging are determined, and MRI evaluations (extent and location of necrosis, and amount of edema) are obtained. Core decompression in precollapsed hips is cost-effective in that it delays THR and prevents painful symptoms for ∼5 years. When coupled with bone graft material such antigen-extracted, autolyzed fibula allograft and purified human bone morphogenetic protein, new bone formation is enhanced and improves clinical outcome [Lieberman JR et al. Clin Orthrop Relat Res 2004]. There is a need to develop more effective therapies for midsize and large lesions.
C. Lowry Barnes, MD, University of Arkansas Medical Sciences, Little Rock, Arkansas, USA, presented a surgical technique he uses to treat AVN in place of standard core decompression. The approach begins similar to traditional core decompression techniques. The lesion is accessed using a 2-cm stab incision. Under fluoroscopic guidance, a 3.2-mm fluted guide-wire is introduced into the lesion, followed by placement of a tissue protector over the guide-wire down to the bone. A core in the femoral head is drilled approximately 5 mm from the endothelial surface. Next a working cannula with an obdurator is placed through the tissue protector and into the core. After removal of the tissue protector and obdurator, the necrosed area is debrided using the curette and/or the fluted guide wire. Advanced debridement of dead bone is performed with the X-REAM Percutaneous Expandable Reamer. After debridement, the reamer is withdrawn and the area is cleaned and filled with biomaterial supplemented with calcium sulphate and calcium phosphate. Final placement of the graft is confirmed under fluoroscopic guidance. This approach allows for expandable decompression of the femoral head to better decompress and debride the necrotic bone. In an ongoing study, Dr. Barnes reported that nearly three fourths of patients treated with this technique responded to treatment without the need for subsequent hip placement and with no complications.
In the final presentation, Carlos J. Lavernia, MD, Larkin Community Hospital, Miami, Florida, USA, said that despite what earlier speakers had said—long-term treatment of osteonecrosis is best achieved with THR.
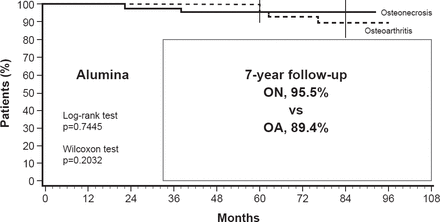
Dr. Lavernia believes the movement to preservation and away from THR was, in part, spurred by early studies that reported high failure rates and variable outcomes with THR. He noted, however, that with improved techniques and materials, recent studies are reporting excellent success rates (Figure 4) [Seyler TM et al. J Bone Joint Surg Am 2006].
Success Rate in Osteonecrosis and Osteoarthritis After Alumina-on-Alumina Bearing Hip Replacement
OA=osteoarthritis; ON=osteonecrosis.
Reproduced from Seyler TM et al. Use of an Alumina-on-Alumina Bearing System in Total Hip Arthroplasty for Osteonecrosis of the Hip. J Bone Joint Surg Am 2006;88(suppl 3):116–125. With permission from the Journal of Bone and Joint Surgery, Inc.
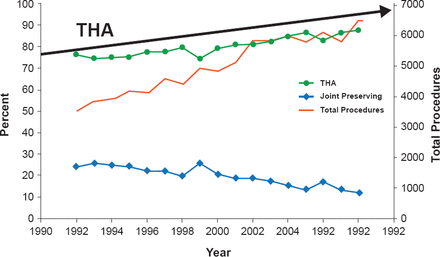
As a result of the improvements, the percentage of THRs performed to treat osteonecrosis of the hip increased from 75% to 88% between 1992 and 2008, while joint-preserving procedures decreased from 25% to 12% during this same period (Figure 5) [Johnson AJ et al. Clin Orthop Relat Res 2014].
Treatment of ONFH in the US: 16-Year Analysis
THA=total hip arthroplasty.
Reproduced from Johnson AJ et al. Treatment of femoral head osteonecrosis in the United States: 16-year analysis of the Nationwide Inpatient Sample. Clin Orthop Relat Res 2014;472(2):617–23. With permission from Springer.
Since 1993, most studies report a THR survival rate of >80% overall and >90% for patients aged ≤55 years. Cemented and uncemented THR results appear comparable after 9.3 years of follow-up [Kim YH et al. J Bone Joint Surg 2003]. While years ago, implant longevity was poor, in 2014 it is expected to be between 25 and 30 years. Based on their physiologic age, Dr. Lavernia will perform a THR in patients with Stage II, III, or IV disease.
The cause of the osteonecrosis is an important determinant of outcome. Patients with sickle-cell anemia for example have a higher rate of complications such as infection and loosening. Patients who have hardware from prior surgery, those with poor bone quality, and those who have stem cell therapy can present challenges. Despite the challenges, Dr. Lavernia believes THR is the best long-term treatment for AVN, even in the very young.
- © 2014 MD Conference Express®