Summary
This article presents results from a double-blind, randomized clinical trial demonstrating that injections of hyaluronic acid were no more effective than placebo in treating patients with knee osteoarthritis.
- Bone Density & Structure Disorders
- Arthritis
- Orthopaedics Clinical Trials
- Arthritis
- Hip & Knee Conditions
- Orthopaedics & Sports Medicine
Walter van der Weegen, MSc, St. Anna Hospital, Geldrop, The Netherlands, presented results from a double-blind, randomized clinical trial demonstrating that injections of hyaluronic acid (HA) were no more effective than placebo in treating patients with knee osteoarthritis (KOA).
Whether the use of synthetic HA injections are helpful for KOA has been the subject of debate for decades. Numerous published reviews on the effectiveness of HA treatment in this patient population have been inconclusive [Rutjes AW et al. Ann Intern Med 2012; Arrich J et al. CMAJ 2005; Reichenbach S et al. Arthritis Rheum 2007; Divine JG et al. Clin Orthop Relat Res 2007; Bellamy N et al. Cochrane Database Syst Rev 2006].
However, data from patients at St. Anna Hospital have demonstrated potential efficacy of HA treatment in KOA. With this in mind, Prof. van der Weegen and colleagues conducted a randomized, double-blind, placebo-controlled trial to collect more information on the effectiveness and safety of HA injections in this patient population. The study aimed to determine whether HA injections would be more effective than placebo for knee pain and function in KOA.
Inclusion criteria included patients with a radiographic score of 1 to 3 based on the Kellgren and Lawrence grading scheme. Patients previously treated with HA were excluded.
Patients (n=200) at two hospitals with mild to moderate KOA received either three intra-articular injections of HA (2 mL injections, 30 mg HA with a molecular weight of 2.2 M Dalton) or placebo at weekly intervals, with follow-up at 1, 3, and 6 months to assess visual analog scale (VAS) scores for pain, Western Ontario and McMaster Universities Arthritis (WOMAC) scores, knee range of motion, treatment satisfaction, and adverse events. Baseline details, including age, KOA severity, and pain scores were similar in both groups.
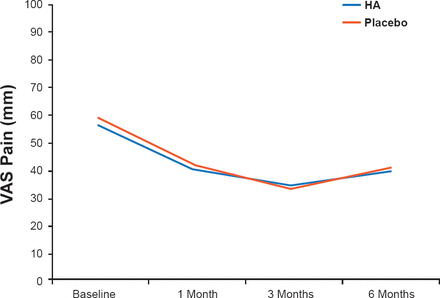
Although patients' pain scores improved significantly from baseline, there was no significant difference between the two groups (Figure 1). Additionally, there was no significant difference between groups at any of the follow-up time intervals in all other outcome measurements (VAS scores for pain, WOMAC scores, knee range of motion, and treatment satisfaction). No serious adverse events were reported in either of the treatment groups.
Visual Analog Scale Scores for Pain
Fermathron plus is the hyaluronic acid compound used in the study.
Reproduced with permission from WA. van der Weegen, MD.
Similarly, although subgroup analyses on pretreatment duration of symptoms, pain severity, and radiological KOA severity were also performed, there were no significant differences between subgroups.
Although an earlier study using the same HA as used in this trial demonstrated clinical improvement from baseline [McDonald C et al. J Clin Res 2000], the results of this current study showed that three injections of HA were no more effective than placebo. Prof. Van der Weegen therefore concluded that HA treatment can not be recommended for patients with mild to moderate knee OA.
- © 2014 MD Conference Express®