Summary
Results of a double-blind, randomized, placebo-controlled, parallel-group trial do not support the routine use of metformin in nondiabetic patients after ST-segment elevation myocardial infarction (STEMI) for the purpose of preserving myocardial function. Findings of the Metformin to Reduce Heart Failure After Myocardial Infarction trial [GIPS-III; Lexis CPH et al. JAMA 2014] are discussed in this article.
- Myocardial Infarction
- Cardiology Clinical Trials
- Heart Failure
- Cardiology & Cardiovascular Medicine
- Myocardial Infarction
- Cardiology Clinical Trials
- Heart Failure
Results of a double-blind, randomized, placebo-controlled, parallel-group trial do not support the routine use of metformin in nondiabetic patients after ST-segment elevation myocardial infarction (STEMI) for the purpose of preserving myocardial function. Findings of the Metformin to Reduce Heart Failure After Myocardial Infarction trial [GIPS-III; Lexis CPH et al. JAMA 2014] were presented by Chris P. H. Lexis, MD, University Medical Center, Groningen, Groningen, The Netherlands.
MI diminishes left ventricular function (LVF) in up to 50% of subjects and leads to clinical heart failure in up to 40% [Steg PG et al. Eur Heart J 2012]. Animal experiments and observational suggest metformin may have protective effects on the myocardium in the setting of ischemia-reperfusion; the mechanism of action being independent of the drug's glucose-lowering effect. The GIPS-III trial tested whether 4 months of metformin treatment started after successful percutaneous coronary intervention (PCI) for MI could preserve left ventricular ejection fraction (LVEF) in patients without diabetes at 4 months.
Acute STEMI patients, post-PCI with stenting, and with TIMI post-PCI flow Grade ≥2 were eligible for the trial. Key exclusion criteria were diabetes, prior MI, prior CABG, need for cardiothoracic surgery, contraindication for magnetic resonance imaging (MRI), and severe renal impairment. Three-hundred and eighty patients were randomized 1:1 to receive metformin 500 mg BID (n=191) or placebo BID (n=189) beginning immediately after PCI and continuing for 4 months. The primary endpoint was the LVEF measured in a blinded fashion at 4 months using a 3.0 Tesla MRI. The principal secondary endpoint was N-terminal brain natriuretic peptide (NT-proBNP) at 4 months. cardiovascular outcomes, adverse events, and markers of glycometabolic state were also collected.
Time from onset of symptoms to first coronary intervention was 161 minutes (IQR, 109–250). Time to administration of first dose of study drug after first coronary intervention was approximately 100 minutes in each arm. The two arms were similar at baseline for demographic and physiologic characteristics including specific angiographic parameters. Angiographic markers of successful reperfusion were less successful for patients receiving metformin versus placebo (TIMI post-PCI flow Grade <3, 12.6% vs 5.3%; myocardial blush Grade ≤1: 13.8% vs 5.9%).
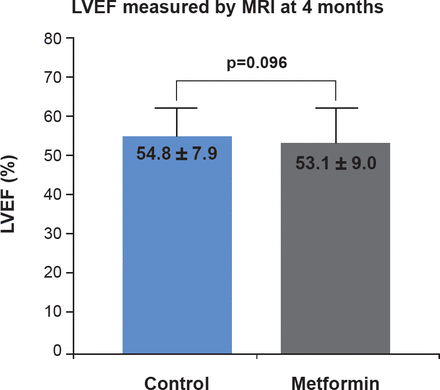
In both arms, over 25% of subjects did not undergo MRI; 55 and 50 patients in the metformin and placebo arms, respectively. For those that had a successful MRI, the primary endpoint of LVEF at 4 months was similar for both arms (53.1±9.0 vs 54.8±7.9; p=0.096; Figure 1), as was the secondary endpoint of NT-proBNP and other relevant laboratory markers (Table 1).
Principal Secondary Endpoint and Laboratory Markers at 4 Months
MRI Determination of LVEF
LVEF=left ventricular ejection fraction; MRI=magnetic resonance imaging.
Reproduced with permission from CPH Lexis, MD.
Adverse events were similar in both arms, with no deaths or episodes of lactic acidosis. There was no evidence of superiority of metformin in subgroup analyses by sex, age, body mass index, MI location, TIMI flow pre-PCI, admission levels of glucose, or NT-proBNP.
Metformin 500 mg twice daily beginning after PCI and continuing for 4 months does not preserve LVEF after STEMI in patients without diabetes. Even though metformin appears to be safe, the current results do not support its routine use in this patient setting.
- © 2014 MD Conference Express®