Summary
In some cases of non—small cell lung cancer (NSCLC), mutations in oncogenes appear to be the underlying mechanism of lung cancer development. Specifically targeted therapies are promising in the treatment of NSCLC, but mutations in oncogenic drivers can result in drug resistance. This article discusses drug resistance to ALK and ROS1 inhibitors, as well as BRAF and HER2 mutations that result in drug resistance.
- Oncology Genomics
- Respiratory Cancers
- Cancer
- Oncology Genomics
- Oncology
- Respiratory Cancers
- Cancer
In some cases of non-small cell lung cancer (NSCLC), mutations in oncogenes appear to be the underlying mechanism of lung cancer development. Specifically targeted therapies are promising in the treatment of NSCLC, but mutations in oncogenic drivers can result in drug resistance. Robert C. Doebele, MD, PhD, University of Colorado Cancer Center, Aurora, Colorado, USA, discussed drug resistance to ALK and ROS1 inhibitors. ALK and ROS1 gene fusions drive about 5% and 1% of lung cancers, respectively.
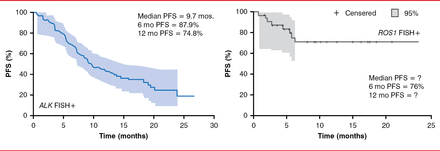
The ALK and ROS1 kinases have high homology, so some inhibitors are effective against both kinases in vitro, such as crizotinib and ceritinib [Davies KD et al. Clin Can Res 2012]. In addition, crizotinib treatment in patients with NSCLC with ALK or ROS1 gene fusions resulted in an objective response rate of 60.8% (95% CI, 52.3 to 68.9) and 56% (95% CI, 24.4 to 65.1), respectively, in 2 separate trials [Camidge DR et al. Lancet Oncol 2012; Ou SH et al. J Clin Oncol 2013. Abstract 8032]. Similarly, the 6-month progression-free survival was 87.9% and 71% in patients with NSCLC with ALK or ROS1 gene fusions (Figure 1) [Camidge DR et al. Lancet Oncol 2012; Ou SH et al. J Clin Oncol 2013]. Crizotinib eventually fails in patients with ALK or ROS1 gene fusions, however, because of drug resistance.
Effect of Crizotinib in Patients With ALK and ROS1 Gene Fusions
PFS=progression-free survival; FISH+=fluorescence in situ hybridization-positive.
Reproduced from Camidge DR et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncology 2012;13(10);1011–1019. With permission from Elsevier.
The mechanisms of crizotinib resistance can be categorized as ALK-dominant mechanisms, such as resistance mutations or lack of adequate central nervous system (CNS) penetration, and ALK nondominant mechanisms, which are primarily a result of bypass signaling [Camidge DR, Doebele RC. Nat Rev Clin Oncol 2012]. Currently, multiple second-generation ALK and ROS1 inhibitors that can potentially overcome resistance are under development [Camidge DR et al. ESMO 2013 3.401]. Some ALK mutations may, however, be resistant to the second-generation ALK inhibitors [Doebele RC et al. JTO 2014].
Similarly, next-generation ROS 1 inhibitors that may overcome resistance are under development. The agents PF-06463922 and foretinib demonstrated activity against ROS1 mutations in vitro [Zou HY et al. AACR 2013; Devare MA et al. Proc Natl Acad Sci USA 2013]. In crizotinib-naïve and -resistant ALK+ patients, treatment with the agent LDK378 resulted in an overall response rate (ORR) of 60% and 57%, respectively [Shaw AT et al. J Clin Oncol 2013]. Similarly, in patients with NSLCL with ALK mutations, treatment with alectinib resulted in an ORR of 54.5% [Ou S et al. ESMO 2013. Abstract 44], and treatment with AP26113 resulted in an ORR of 61% [Camidge DR et al. ESMO 2013 3.401].
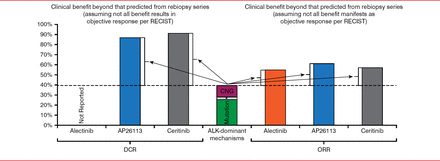
Dr. Doebele pointed out that there appears to be a disconnect between the mechanisms believed to drive drug resistance and the response to second-generation ALK and ROS1 inhibitors. Considering that the approximately 30% of patients with NSCLC with ALK inhibitor-resistant mutations will not all respond to therapy, and some will have mutations that are expected to be resistant to the second-generation inhibitors, the response to these agents is far better than expected (Figure 2) [Doebele RC. J Thorac Oncol 2014]. The reasons for this are unknown, but it maybe a result of having missed mutations in patient samples, resensitization, and targeting of bypass pathways by these new inhibitors.
Better Than Expected Response to Second-Generation ALK and ROS1 Inhibitors
RECIST=response evaluation criteria in solid tumors; DCR=disease control rate; ORR=overall response rate; CNG=copy number gain.
Reproduced with permission from RC Doebele, MD, PhD.
Benjamin Besse, MD, PhD, Gustave Roussy Cancer Campus Grand Paris, Villejuif, France, discussed BRAF and HER2 mutations that result in drug resistance. The incidence of BRAF mutations is about 1.7%, with HER2 mutations even lower at 0.9% [Barlesi F et al. J Clin Oncol 2013]. It is not clear if BRAF mutations (specifically, the V600E mutation) are a negative prognostic factor. Two studies of patients with resected NSCLC, however, demonstrated that BRAF mutations resulted in lower overall survival compared with patients with wild-type BRAF [Kinno T et al. Ann Oncol 2014; Marchetti A et al. J Clin Oncol 2011].
The first BRAF inhibitor, vemurafenib, demonstrated promising results in melanoma with an improvement in progression-free survival (PFS) [Flaherty KT et al. N Engl J Med 2010]. The second BRAF was dabrafenib, which also showed the promising results of an improvement in PFS in melanoma [Hauschild A et al. Lancet 2012]. In addition, a Phase 2, single-arm, open-label trial of dabrafenib in patients with BRAF V600E-mutation NSCLC is currently underway, with a primary objective of increasing the ORR [NCT01336634]. Data from the first 20 patients indicate that the ORR is 40%, with a majority of the patients demonstrating partial response [Planchard D et al. J Clin Oncol 2013]. Although PFS is not available, the median duration of treatment was 84 days, with some patients receiving treatment for more than 1 year. Common adverse events included fatigue (40%), decreased appetite (32%), asthenia (24%), rash (24%), nausea (24%), diarrhea (20%), anemia (24%), and hypophosphatemia (12%). Importantly, patients must be followed closely for induction of other cancers, such as squamous cell carcinoma of the skin. The dabrafenib trial design was amended to allow recruitment of an additional cohort of patients who were treated with combination therapy of dabrafenib and the MEK inhibitor trametinib, and it demonstrated that PFS was improved with combination therapy versus monotherapy.
NSCLC driven by HER2 mutations is rare, but it appears to occur mostly in women who were never smokers [Mazieres J et al. J Clin Oncol 2013]. These investigators also showed that in patients with stage IV lung cancer with HER2 mutations, the disease control rate was 93% with trastuzumab treatment and 100% with afatinib treatment. The results of Phase 2 trials that are underway are awaited, including a Phase 2 trial in patients with stage IV NSCLC with HER2 mutations who were pretreated and randomized to neratinib monotherapy or neratinib plus temsirolimus. The European Thoracic Oncology Platform trial will soon begin to recruit the same type of population for the evaluation of afatinib.
In conclusion, although drug resistance is a challenge with targeted therapies for treating NSCLC, the next-generation treatments that are being tested may potentially overcome resistance caused by many mutations.
- © 2014 MD Conference Express®