Summary
In the literature, pathology is described as having revolutionized the management of lung cancer, yet the current understanding of lung cancer pathology has also presented new challenges. This article discusses considerations of molecular testing that is currently used in clinical practice.
- Respiratory Cancers Genomics
- Cancer
- Oncology
- Respiratory Cancers
- Oncology Genomics
- Cancer
In the literature, pathology is described as having revolutionized the management of lung cancer, yet the current understanding of lung cancer pathology has also presented new challenges. Keith M. Kerr, MD, Aberdeen University Medical School, Aberdeen, United Kingdom, presented considerations of molecular testing that is currently used in clinical practice.
Several studies suggest that finding actionable genetic alterations, which are mutations that can be targeted by therapy, is a worthwhile endeavor in lung cancer. In a trial conducted by the Lung Cancer Mutation Consortium, a survival benefit was clearly seen in patients who received driver detected-targeted therapy compared with patients who did not have genetic drivers detected and in those who did have drivers detected but did not receive targeted therapy [Kris MG et al. WCLC Sydney 2013]. This effect resulted primarily from mutations in epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK). Dr. Kerr pointed out that this benefit was not due to a prognostic factor related to the genetic driver, as patients with lung cancer driven by genetic aberrations who did not receive targeted therapy had similar overall survival as those who did not have genetic drivers. Pathology can now stratify lung adenocarcinoma into disease driven by EGFR mutations or by ALK or ROS1 rearrangements. These 3 genetic alterations can be targeted by specific therapies.
Histologic subtyping of non-small cell lung cancer (NSCLC) can be accurately achieved by using simple hematoxylin and eosin staining of a small biopsy or cytology sample. There are cases, however, in which no differentiating features can be seen in the sample, which is designated as NSCLC not otherwise specified. Many times, this designation is made because only a few cancerous cells are present in the sample. However, immunohistochemistry (IHC) can then be used to determine if there are specific genetic markers that can lead to a more differential diagnosis. This approach has decreased the numbers of biopsy and, especially cytology samples that receive diagnoses of NSCLC not otherwise specified. However, Dr. Kerr pointed out, it is important to understand that although these molecular markers predict the subtype of NSCLC, they do not define the disease. Therefore, a pathologist may use the term “probably” when IHC is used to determine the subtype of NSCLC.
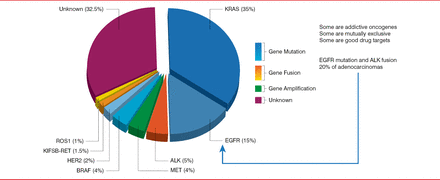
In lung adenocarcinoma in Europe, EGFR is a driver in about 15% of cases, whereas ALK is a driver in about 5% (Figure 1) [Kerr KM. J Clin Pathol 2013]. Molecular drivers are closely associated with adenocarcinoma histology and adenocarcinogenesis. Therefore, all samples classified as adenocarcinoma, probably adenocarcinoma, partly adenocarcinoma, or “cannot exclude” adenocarcinoma should be tested for molecular markers. In addition, about half of patients with factors such as smoking, male sex, and certain ethnic heritages are likely to have one of these molecular markers. Therefore, patients who do not fit the typical clinical profile but have one of these characteristics should also undergo molecular testing. Furthermore, many guidelines also suggest that patients who never smoked or are long-time ex-smokers should also receive molecular testing.
Proportion of Oncogenic Drivers in Lung Adenocarcinoma
ALK=anaplastic lymphoma kinase; EGFR=epidermal growth factor receptor
Reproduced from Kerr KM et al. Clinical relevance of the new IASLC/ERS/ATS adenocarcinoma classification. J Clin Pathol 2013;66(10):8320–8. With permission from the British Medical Journal Publishing Group.
Depending on the hospital, sometimes molecular testing of lung tumors is initiated by the pathologist or ordered by the oncologist (or tumor board). The advantages of a pathologist's initiating molecular testing are that testing occurs quickly, cases are less likely to be missed because molecular testing is routine, and the results are ready for tumor board decisions. However, disadvantages include the potential for wasted time, money, and tumor tissue, because some results of molecular testing will never be acted on. In contrast, the advantages of molecular testing initiated by an oncologist include testing that is performed only when needed, preservation of tissue, and no wasted laboratory time. However, the disadvantages are that the cost of testing is higher per sample, the turnaround for results is slower, and cases maybe missed.
Molecular testing can be performed on tissue or cytology cell block sections, but it is critical that an appropriate proportion of tumor and normal cells be present in the sample. The appropriate proportion of tumor cells is approximately 10% to >50% of the sample. However, Prof. Kerr pointed out that it is very difficult to establish a specific amount or number of tumor cells that are needed for molecular testing especially for IHC or fluorescence in situ hybridization. Clearly, a tissue sample taken from a resection provides abundant tumor cells, whereas a lung biopsy provides less material, and a cell pellet harvested from a cytology sample provides the least material for molecular testing. Importantly, only about 33% to 50% of a malignant bronchial biopsy sample is tumor cells [Coghlin CL et al. J Thorac Oncol 2010].
Preparation of the sample is an important part of molecular testing. In particular, proper fixing of the tissue is critical: over- or underfixing of the tissue will lead to an unsuccessful test. Another important factor in sample processing is the amount of material; in particular, the laboratory performing the deoxyribonucleic acid extraction for molecular testing will need to know the quality and amount of material that is provided. A pathologist may mark the area of a fixed section, indicating where deoxyribonucleic acid should be extracted in an effort to “purify” the sample to increase the proportion of tumor cells. Prof. Kerr stated that this extra “purification” step makes a large difference in achieving successful testing.
Multiple mutations can occur in EGFR. Some of these mutations result in different degrees of drug sensitivity, whereas other mutations may be associated with drug resistance [Sharma SV et al. Nat Rev Cancer 2007]. Therefore, it is important that the molecular testing that is performed adequately cover a large range of EGFR mutations. For testing with ALK rearrangements, fluorescence in situ hybridization is the gold standard, as it illustrates the presence of a rearrangement, although it does not guarantee that the rearrangement is active. IHC can be used to identify elevated levels of the ALK rearrangement protein, and multiplex polymerase chain reaction can identify the increasing number of types of ALK rearrangements. Many pathology laboratories now use IHC to prescreen samples for ALK rearrangement before moving on to fluorescence in situ hybridization in patients with elevated ALK protein levels.
Prof. Kerr outlined the testing algorithm that is used in his pathology laboratory, which includes subtyping malignant lung tumors and then simultaneously testing adenocarcinomas for molecular markers such as EGFR and ALK rearrangements, as well as KRAS and BRAF mutations. Some laboratories use a sequential approach to molecular testing; however, a recent study suggested that with this method, 30% of samples could not undergo required testing [Buettner R et al. J Clin Oncol 2013].
In conclusion, Prof. Kerr highlighted that it is important that clinicians be aware that molecular testing may be required and that there is the need to safely maximize tissue collection. In addition, a multidisciplinary effort is needed to successfully perform molecular testing.
- © 2014 MD Conference Express®