Summary
Multiple novel therapies have been developed to prevent and treat headaches, many of which target specific points in the pathophysiology of the disorder. This article discusses updates in the understanding of the pathophysiology of headaches, new and emerging treatments for migraine headache, trigeminal autonomic cephalalgias, and the treatment of migraine headaches in children.
- Episodic & Paroxysmal Disorders
- Neurology
- Episodic & Paroxysmal Disorders
Multiple novel therapies have been developed to prevent and treat headaches, many of which target specific points in the pathophysiology of the disorder. Andrew Charles, MD, University of California, Los Angeles, Los Angeles, California, USA, discussed updates in the understanding of the pathophysiology of headaches.
A common concept of migraine headache that patients frequently have is the vascular hypothesis, which states that vasodilation within the brain causes migraine headaches. However, multiple lines of evidence indicate that vasodilation of blood vessels within the brain is not sufficient or necessary to cause a migraine [Rahmann A et al. Cephalalgia 2008; Schoonman GG et al. Brain 2008; Kruuse C et al. Brain 2003]. For example, nitroglycerin or sildenafil can cause migraine as a side effect; however, when migraine is induced by these drugs, cerebral and meningeal blood vessels are not dilated [Schoonman GG et al. Brain 2008; Kruuse C et al. Brain 2003]. Conversely, vasodilators such as vasoactive intestinal peptide do not cause migraine [Rahmann A et al. Cephalagia 2008]. Furthermore, in a study of 19 patients with spontaneous migraine, magnetic resonance angiography demonstrated that there was no large vessel extracranial artery dilation during the migraine [Amin FM et al. Cephalalgia 2013]. Although there was slight intracranial artery dilation during the migraine, sumatriptan was an effective treatment, yet it did not cause intracranial vasoconstriction.
Another suggested paradigm as an underlying mechanism for migraine headache is the neurogenic inflammation hypothesis, which states that migraine is the result of inflammation within the brain that is caused by stimulation of neurons and characterized by vasodilation, vascular permeability, mast cell degranulation, and the release of substance P and calcitonin gene-related peptide (CGRP). Despite a lack of evidence, a third hypothesis of migraine headache is that peripheral trigeminal input causes the classic pain associated with migraines. Additionally, cortical spreading depression (CSD) also been hypothesized to initiate a migraine attack. However, many patients have multiple symptoms during the premonitory phase, such as yawning, fatigue, mood change, and light and sound sensitivity, prior to the onset of the pain associated with migraine. Dr. Charles noted that these symptoms challenge the CSD hypothesis, as it is clear that clinical symptoms occur prior to the onset of the headache.
Although aura is thought to precede the migraine headache, a recent prospective study in which patients recorded their symptoms as they occurred demonstrated that symptoms such as photophobia, phonophobia, and nausea start at the time of aura [Hansen JM et al. Neurology 2012]. Dr. Charles highlighted that migraine does not occur in a linear progression, from one brain region to another. It is a pathologic brain state that involves multiple brain regions and manifests differently in individual patients.
A recently proposed hypothesis is that central sensitization is a secondary process instead of an initiating process. A recent study demonstrated that the hypothalamus, among other regions, is activated during the premonitory phase of migraine [Maniyar FH et al. Brain 2014]. In addition, there appears to be a change in the function of the thalamus during a migraine [Burstein R et al. Ann Neurol 2010; Coppola G et al. Brain 2005]. A final proposed hypothesis is that neurons are a major mediator in the neurological symptoms of migraine. However, Dr. Charles pointed out that multiple cells in the brain are likely responsible for the neurologic symptoms.
Ana Recober-Montilla, MD, University of Iowa Hospitals and Clinics, Iowa City, Iowa, USA, outlined new and emerging treatments for migraine headache. There are several new therapeutic targets including CGRP receptor antagonists anti-CGRP antibodies, 5-HT1F agonists, glial modulation, AMPA/kainate receptor antagonists, orexin receptor antagonists, neuronal nitric oxide synthase (nNOS) inhibitors, and acid-sensing ion channel 1 blockade.
New treatment strategies for migraine include transcranial magnetic stimulation (sTMS), in which a brief, magnetic pulse is applied to the scalp and underlying cortex in an effort to disrupt CSD [Dodick DW et al. Headache 2010; Lipton RB et al. Lancet Neurol 2010]. In a randomized, controlled trial, sTMS and sham treatments were well tolerated but did not improve migraine with aura symptoms. However, one TMS system was recently approved by the US Food and Drug Administration (FDA), and another is awaiting approval.
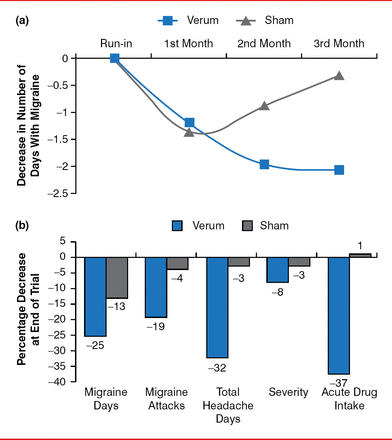
Another new methodology for migraine treatment is supraorbital transcutaneous nerve stimulation, which was recently approved by the FDA. In a randomized, double-blinded, controlled trial, 67 patients with migraine with or without aura received verum neurostimulation or sham for 20 minutes. About 50% of the neurostimulation arm responded with a change in monthly migraine days (Figure 1) [Schoenen J et al. Neurology 2013]. However, neurostimulation did not affect the severity of the pain or other associated symptoms. Despite that, 70% of participants reported that they were either moderately or very satisfied.
Effect of Supraorbital Transcutaneous Nerve Stimulation on Migraine
Reproduced from Schoenen J et al. Migraine prevention with a supraorbital transcutaneous stimulator. Neurology 2013;80(8):697–704. With permission from Lippincott Williams & Wilkins, Inc.
Vagus nerve stimulation is another new strategy for the treatment of migraine. In a pilot study, 2 consecutive doses 90 seconds in duration spaced apart by 15 minutes were applied to 30 patients with migraine [Goadsby PJ et al. Neurology 2013]. For patients who had mild pain at baseline, 63% were pain free by 2 hours, whereas 21% of patients with moderate to severe pain at baseline were pain free by 2 hours.
Other new strategies that have been evaluated for migraine include sphenopalatine ganglion stimulation (SPG), which may abort a migraine attack, and occipital nerve stimulation, which showed disappointing results in 3 randomized controlled trials [Silberstein SD et al. Cephalalgia 2012; Saper IR et al. Cephalalgia 2011]. Another strategy is plastic surgery to decompress the cranial nerves. In a sham-controlled trial, 57% of patients followed for 12 months demonstrated complete elimination of migraine compared with sham treatment, and 87% of patients treated with nerve decompression demonstrated a substantial improvement compared with 58% of patients treated with sham [Guyuron B et al. Plast Reconst Surg 2009].
New delivery systems of current treatments for migraine were also discussed. A new method of delivery for sumatriptan is intranasal delivery of a powder, which is currently under evaluation by the FDA [Diupesland PG et al. Headache 2013]. Another novel delivery system for sumatriptan is through a transdermal patch, which was approved by the FDA in 2013. Although orally inhaled dihydroergotamine (DHE) demonstrated improved tolerability over intravenous DHE, the FDA declined approval in 2013 [Aurora SK et al. Headache 2011; Cook RO et al. Headache 2009].
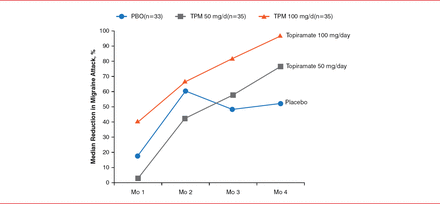
Amy Gelfand, MD, University of California at San Francisco, San Francisco, California, USA, discussed the treatment of migraine headaches in children. Topiramate, which was the first FDA-approved migraine prophylactic for use in children aged 12 to 17 years, was approved in 2014. During a study, patients aged 12 to 17 years with episodic migraine were randomly assigned to receive 50 mg QD or 50 mg BID of topiramate or placebo [Lewis D et al. Pediatrics 2009]. Patients treated with 50 mg BID of topiramate demonstrated a significant reduction in migraine attacks compared with placebo (p=0.02; Figure 2).
Reduction of Migraine Attacks With Topiramate in Children
Mo=month; PBO=placebo; TPM=topiramate.
Reproduced from Lewis D et al. Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics 2009;123(3):924–934. With permission from the American Academy of Pediatrics.
There are currently 2 agents, almotriptan and rizatriptan, that are approved for the treatment of acute migraine in children. A study of cognitive behavioral therapy plus migraine preventive therapy (amitriptyline) was conducted in 10- to 17-year-olds with chronic migraine [Powers SW et al. JAMA 2013]. All participants received amitriptyline and were then randomly assigned to receive cognitive behavioral therapy (CBT) or headache education. There was a significant reduction in days of headache and headache disability during the 20-week treatment period in patients who underwent CBT compared with headache education (p=0.002 and p<0.001, respectively).
Peter I. Goadsby, MD, University of California, San Francisco, San Francisco, California, USA, discussed trigeminal autonomic cephalalgias (TACs), which includes cluster headache, paroxysmal hemicrania, and short-lasting unilateral neuralgiform headache attack with conjunctival injection and tearing (SUNCT).
In a randomized, controlled trial with patients with cluster headache, researchers demonstrated that treatment with oxygen resulted in pain-free and associated symptom-free response rates of 78% and 66%, respectively, compared with 20% and 31%, respectively, in the placebo arm [Cohen AS et al. JAMA 2009]. The efficacy of oxygen is likely a result of inhibition of trigeminal neurons, as oxygen inhibits blood flow to the lacrimal sac [Akerman S et al. Headache 2009]. For the short-term prevention of cluster headache, a greater occipital nerve (GON) injection with lidocaine (2%) plus 80 mg of methylprednisolone resulted in a response rate of 60% [Afridi SK et al. Pain 2006]. For the long-term prevention of cluster headache, up to 960 mg QD of verapamil is effective; however, in addition to constipation, leg swelling, and gingival hyperplasia, verapamil can cause slowing of the A-H interval and arrhythmias (Table 1) [Cohen AS et al. Neurology 2007; Matharu MS et al. Neurol Neurosurg Psychiatry 2005]. Surgical management of cluster headaches includes stimulation of the sphenopalatineganglion, which achieved pain relief in more than 50% of attacks in 7 of 28 patients, while 10 patients experienced fewer attacks [Schoenen J et al. Cephalalgia 2013]. Similarly, occipital nerve stimulation resulted in moderate to markedly improved outcomes in 6 of 22 patients with cluster headache [Burns B et al. Lancet 2007]. In addition, noninvasive vagus nerve stimulation resulted in a pain free response rate of 21% at 24 hours and pain relief in 42% of patients with episodic migraine [Goadsby et al. Cephalalgia 2014 In press].
Cardiovascular Effects of Verapamil
Expanding our understanding of the pathophysiology of migraine and other headaches has led to the development of novel therapies for the prevention and treatment of headaches.
- © 2014 MD Conference Express®