Summary
Atrial fibrillation (AF) is increasing in incidence in the United States [Go AS et al. JAMA 2001], and its presence increases the risk of developing stroke 5-fold [H-J Lin et al. Stroke 1996]. In addition, patients with AF tend to experience more severe strokes and are more likely to have a recurrent stroke. This article discusses the detection of occult AF in patients with cryptogenic stroke, selecting oral anticoagulants, the prevention of cardioembolic stroke in special populations, as well as provides an overview of risk stratification of stroke in patients with AF.
- Arrhythmias
- Cerebrovascular Disease
- Neurology
- Arrhythmias
- Cerebrovascular Disease
Atrial fibrillation (AF) is increasing in incidence in the United States [Go AS et al. JAMA 2001], and its presence increases the risk of developing stroke 5-fold [H-J Lin et al. Stroke 1996]. In addition, patients with AF tend to experience more severe strokes and are more likely to have a recurrent stroke. The use of appropriate anticoagulation with warfarin [Hart RG et al. Ann Intern Med 2007] or the novel anticoagulants [Ruff CT et al. Lancet 2014] reduces the risk of stroke. However, patients with atrial fibrillation may be asymptomatic. In this population, appropriate anticoagulation is underutilized. Steven Messé, MD, University of Pennsylvania, Philadelphia, Pennsylvania, USA, discussed the detection of occult AF in patients with cryptogenic stroke.
In some cases, cryptogenic stroke maybe a result of AF. One study demonstrated that 28% of patients with cryptogenic stroke had AF [Tayal AH et al. Neurology 2008]. In this study, AF was determined in less than 30 seconds in 23% of the patients; however, in other patients, AF was not detected until days after monitoring was initiated. In a retrospective, consecutive cohort study, patients with cryptogenic stroke or transient ischemic attack (TIA) were monitored for 28 days with mobile cardiac outpatient telemetry (MCOT) [Kasner S et al. International Stroke Conference (ISC) 2014]. AF was detected in 14% of patients, with 46% of AF episodes detected being less than 30 seconds duration. Patients with prior cortical or cerebellar infarct and age >60 years were at an increased risk of AR Dr. Messé indicated that these studies suggest that MCOT is able to detect AF, which may be the underlying cause for a substantial proportion of cryptogenic stroke.
Another method of AF detection was evaluated by the EMBRACE trial, in which 572 patients aged ≥55 years with cryptogenic stroke or TIA received a 24-hour Holter monitor in the hospital, and were then randomly assigned to either an additional 24 hours of Holter monitoring or 30 days of continuous monitoring with a dry electrode event-triggered loop recorder [Gladstone D et al. ISC 2013]. Continuous monitoring led to the detection of AF in 16% of patients compared with 3% of patients with Holter monitoring. AF events lasted for <2.5 minutes in about 30% of patients, 5 minutes in 30% of patients, and about 25% of patients had an episode that lasted for >10 minutes.
The SURPRISE! study evaluated an implantable loop recorder in 85 patients who were monitored for a mean of 569 days. Paroxysmal AF was detected in 20.7% of patients, and importantly, most cases were asymptomatic, with episodes lasting between 1 and 4 hours. The prospective, randomized CRYSTAL AF study evaluated a subcutaneous cardiac monitor in 450 patients with cryptogenic stroke [Bernstein R et al. ISC 2014] At 6 months, AF was detected in 8.9% of patients with the implanted monitor compared with 1.4% in the control arm (HR, 6.43; 95% CI, 1.90 to 21.74; p=0.0006). In addition, by 12 and 36 months, AF was detected in 12.4% and 30% of patients compared with 2% and 3% in the control arm. Explantation of the cardiac monitor was required in 2.4% of patients.
Gary S. Gronseth, MD, University of Kansas Medical Center, Kansas City, Kansas, USA, outlined the selection of oral anticoagulants (OACs) for the prevention of cardioembolic stroke in patients with nonvalvular AF. The RE-LY, ROCKET, ARISTOTLE, and ENGAGE AF-TIMI 48 trials demonstrated that treatment with dabigatran, rivaroxaban, apixaban, and edoxaban, respectively, resulted in a decreased risk of ischemic stroke and systemic embolism compared with warfarin [Giugliano RP et al. N Engl J Med 2013; Granger CB et al. N Engl J Med 2011; Patel MR et al. N Engl J Med 2011; Connolly SJ et al. N Engl J Med 2009]. This risk reduction was statistically significant only for dabigatran. However, they also resulted in an increased risk of gastrointestinal (GI) hemorrhage. For example, a meta-analysis found that the hazard ratio favors warfarin for all agents except apixaban in regard to GI hemorrhage.
Some experts prefer the newer OACs over warfarin due to a lower risk of death and intracranial hemorrhage. However, some clinicians have concerns about how to monitor the newer agents and the ability to reverse anticoagulation. The effect of warfarin is monitored by the international normalized ratio (INR); however, this cannot be used with the novel OACs. Instead, prothrombin time and partial thromboplastin time are able to qualitatively assess for the effects of direct factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) and direct thrombin inhibitors (dabigatran), respectively. Currently, there are no antidotes widely available for the novel OACs. Thus, reversal of their anticoagulant effects depends on their elimination from the body.
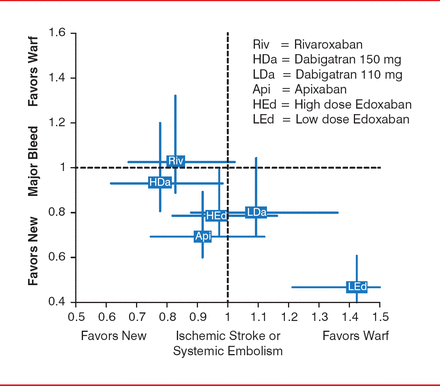
There are no head-to-head trials comparing the novel OACs to one another, only indirect data based on the trials that used warfarin as an active comparator. Dr. Gronseth highlighted a study that used indirect data to map the favorability of the novel OACs in the prevention of ischemic stroke or systemic embolism, as well as bleeding risk in patients with nonvalvular AF (Figure 1). An indirect analysis of the efficacy and safety of the novel OACs compared to warfarin suggests that the novel OACs fall into two clusters: novel OACs that are superior to warfarin for preventing AF embolic complications but have an overall bleeding risk comparable to warfarin (rivaroxaban and high-dose dabigatran) and novel OACs that are safer than warfarin relative to overall bleeding risk but reduce AF embolic complications at a rate comparable to warfarin (apixaban and high-dose edoxaban).
Indirect Comparison of the New Oral Anticoagulants
Reproduced with permission from Gary S. Gronseth, MD.
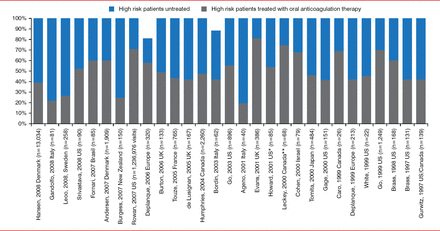
Carlos S. Kase, MD, Boston Medical Center, Boston, Massachusetts, USA, discussed the prevention of cardioembolic stroke in special populations. Despite the well-known risk of stroke in patients with AF and the efficacy of its prevention with OACs, the use of OACs remains low (Figure 2) [Ogilvie IM et al. Am J Med 2010]. Reasons for this in some patients maybe advanced age, dementia, and chronic kidney disease, as well as a history of falls or bleeding tendency.
Underuse of Oral Anticoagulation in Patients With Atrial Fibrillation
Reproduced from Ogilvie IM et al. Underuse of Oral Anticoagulants in Atrial Fibrillation: A Systematic Review. Am J Med. 2010;123(7):638–645.e4. With permission from Elsevier.
*On December 1, 2014, this was changed from Canada to US. On December 1, 2014 this was changed from US to Canada.
The prevalence of AF increases as age advances, so that about 5% of patients aged 70 to 79 years and 9% of patients aged 80 to 89 years have AF [Feinberg WM et al. Arch Intern Med 1995; Wolf PA et al. Stroke 1991]. Warfarin reduces the risk of stroke in patients with AF of all ages, and its benefit is particularly strong in the older than 70 years group, as they have the highest risk of AF-related stroke [van Walraven C et al. Stroke 2009]. Although the rates of serious bleeding in patients taking warfarin increase with advancing age, the magnitude of the effect on stroke prevention exceeds that of the serious bleeding risk.
Similarly, patients of advanced age are more likely to have dementia as well. Patients with Alzheimer-type dementia are at risk of developing cerebral amyloid angiopathy (CAA), which can cause lobar intracerebral hemorrhage (ICH). Therefore, patients with dementia who are receiving OACs are potentially at a greater risk of developing ICH. The elderly are also prone to falls, and a history of fall poses a concern of traumatic intracranial hemorrhage (both ICH and subdural hematoma). Although patients with AF at risk of falls are significantly more likely to experience traumatic intracranial hemorrhage (p<0.0001) than those without falls, those with a CHADS2 score of 2 to 6 also have a significant reduction in the risk of ischemic vascular events with OAC therapy (HR, 0.75; 95% CI, 0.61 to 0.91; p=0.004) [Gage BF et al. Am J Med 2005]. In addition, a Markov decision analysis that took into consideration the risk of traumatic intracranial hemorrhage in patients prone to falling showed that the accrual of quality-adjusted life years (QALYs) was higher in those treated with warfarin compared with those who were not treated with antithrombotic agents [Man-Son-Hing M et al. Arch Intern Med 1999].
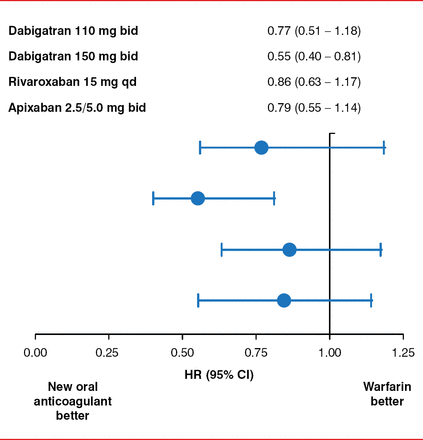
Another possible limiting factor in the administration of OACs is chronic kidney disease (CKD), as about 30% of patients with AF have CKD. Many of the novel OACs are primarily cleared through the kidneys and require a dose adjustment in the setting of renal impairment. Despite this, recent randomized clinical trials found that the novel OACs, rivaroxaban, apixaban, and especially dabigatran, were favorable over warfarin for the prevention of stroke in patients with AF and stage III CKD (Figure 3) [Hart RG et al. Can J Cardiol 2013].
Use of the Novel Oral Anticoagulants in Chronic Kidney Disease
bid=twice daily; qd=once daily.
Reproduced from Hart RG et al. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiology 2013; Jul;29(7 Suppl):S71–S78. With permission from Elsevier.
Seemant Chaturvedi, MD, Wayne State University, Detroit, Michigan, USA, provided an overview of risk stratification of stroke in patients with AF. Analysis of data from the National Registry of Atrial Fibrillation led to the development of the CHADS2 risk score, in which 1 point each is assigned to recent congestive heart failure (CHF), history of hypertension, age ≥75 years, and diabetes, and 2 points assigned to history of stroke or TIA [Gage GF et al. JAMA 2001]. Limitations in the CHADS2 score led to the development of the CHADS2 VASc risk score (which includes gender), in which 1 point is assigned to CHF, hypertension, diabetes, vascular disease, age 65 to 74 years, and female sex, and 2 points are assigned to age >75 years, and previous stroke/TIA/systemic embolism. The 1-year rate of stroke or thromboembolism in patients with a CHADS2 VASc score of 0 ranges from 0.84 to 8.18 in patients with a score of 4 [Banerjee A et al. Thromb Haemost 2012]. A meta-analysis demonstrated that the CHADS2 VASc score had the lowest number of patients stratified as low-risk for stroke who went on to experience events compared with other risk scores including CHADS2 and Framingham [Aakre CA et al. Stroke 2014]. Risk of bleeding can also be determined through the use of risk scores. HAS-BLED is a risk score that assigns 1 point to hypertension, stroke, bleeding, labile INR, and elderly age and 1 or 2 points for abnormal renal or liver function and drug or alcohol use [Pisters R et al. Chest 2010].
Another risk factor for AF that is under-recognized is sleep apnea. Interestingly, AF occurs in about 5% of patients with severe obstructive sleep apnea (OSA) compared with 1% in patients without OSA. In addition, nocturnal oxygen saturation was predictive of new onset AF in a cohort of patients without AF who underwent polysomnography and were followed for 5 years. [Gami AS et al. J Am Col Cardiol 2007]
Patients with AF are at a greater risk of developing stroke; however, appropriate therapy with OACs—warfarin or the newer agents such as dabigatran, rivaroxaban, apixaban, and edoxaban—can substantially reduce the risk. Important considerations should be taken in special populations, such as the elderly, where the presence of comorbidities such as dementia and CKD have led to the under utilization of OACs.
- © 2014 MD Conference Express®