Summary
This article discusses current recommendations for lung cancer screening, as well as some of the similarities and differences between the clinical practice guidelines of the European Society for Medical Oncology (ESMO), American Society of Clinical Oncology (ASCO), and National Comprehensive Cancer Network (NCCN) for treatment of lung cancer. This article also compares these guidelines for early-stage and locally advanced non—small cell lung cancer (NSCLC), demonstrating that despite their similarities, there are often subtle differences in their recommendations.
- Smoking Cessation
- Cancer Guidelines
- Respiratory Cancers
- Smoking Cessation
- Oncology
- Cancer
- Oncology Guidelines
- Respiratory Cancers
James L. Mulshine, MD, Rush University Medical Center, Chicago, Illinois, USA, launched a series of presentations that discussed current recommendations for lung cancer screening, as well as some of the similarities and differences between the clinical practice guidelines of the European Society for Medical Oncology (ESMO), American Society of Clinical Oncology (ASCO), and National Comprehensive Cancer Network (NCCN) for treatment of lung cancer.
SCREENING GUIDELINES FOR NON-SMALL CELL LUNG CANCER (NSCLC)
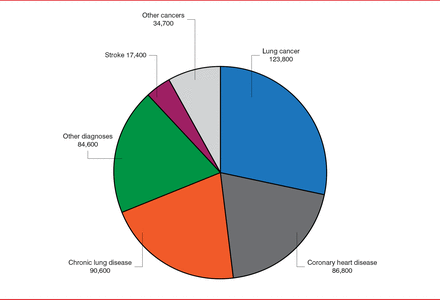
Lung cancer is the most common of all annual tobacco-related deaths (Figure 1). Yet despite the declining rate of heavy smoking in the United States, more than 90 million current or former smokers remain at risk for lung cancer. Because the introduction of low-dose spiral computed tomography (CT) has enhanced the ability to detect lung cancer early, and therefore increased its curability, researchers conducting the National Lung Screening Trial (NLST) sought to determine whether screening with CT could reduce lung cancer mortality.
Causes of Tobacco-Related Death
Source: CDC. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 1997–2001. MMWR 2005;54(25):6 25–628.
This was a randomized, multicenter trial involving more than 53,000 participants (55 to 74 years of age) who were current or former smokers with a history of at least 30 pack-years, and were considered to be at high risk of lung cancer. Participants were randomly assigned to receive either CT or chest radiography (CXR) at 3 annual screenings (baseline, Year 1, and Year 2), with follow-up throughout 6 years. A positive screening was obtained in 24.2% and 6.9% of the CT and CXR groups, respectively, and the rate of false positives was 96.4% and 94.5%, respectively.
The incidence of lung cancer was higher in the CT group compared with the CXR group (645 cases per 100,000 person-years [1,060 cancers] vs 572 cases per 100,000 person-years [941 cases]; risk ratio, 1.13; 95% CI, 1.03 to 1.23). The incidence of death was lower in the CT group compared with the CXR group (247 vs 309 per 100,000 person-years), demonstrating that patients screened with low-dose CT had a 20% lower risk of dying from lung cancer than those screened by CXR (95% CI, 6.8 to 26.7; p=0.004). The rate of death from any cause was also reduced by 6.7% in the CT group (95% CI, 1.2 to 13.6; p=0.02) [National Lung Screening Trial Research Team. New Engl J Med 2011].
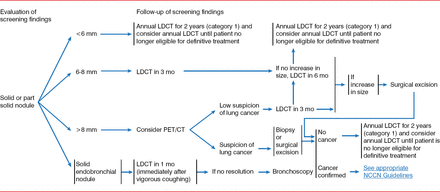
The results from the NLST also formed much of the basis for the recently updated guidelines on lung cancer screening published by the United States Preventive Services Task Force (USPSTF). These guidelines recommend annual lung cancer screening with low-dose CT in asymptomatic, average- or high-risk patients (55 to 79 years) with a 30 pack-year history of smoking who currently smoke or stopped within the past 15 years [Moyer VA and US Preventive Services Task Force. Ann Intern Med 2014]. The NCCN guidelines provide a management schema for the evaluation of findings from screenings (Figure 2).
Management of Screening Results: Recommendations from the National Comprehensive Cancer Network
LDCT=low-dose computed tomography; PET/CT=positron emission tomography/computed tomography.
Source: National Comprehensive Cancer Network.
Despite the benefits of lung cancer screening, however, the USPSTF emphasized that lung cancer is still a tobacco-related disease, and lung cancer screening cannot help with the many other tobacco-related causes of death beyond lung cancer.
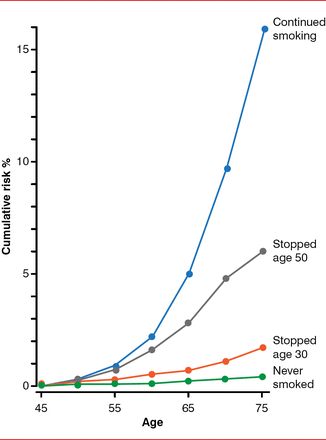
Although continued smoking results in a markedly elevated risk of lung cancer throughout time, more than half of new lung cancers arise in individuals who stopped smoking. Even when a patient has stopped smoking for decades, lung cancer risk does not return to the never-smoker level (Figure 3) [Vineis P et al. J Natl Cancer Inst 2004].
The Lifelong Risk of Lung Cancer After Stopping Smoking
Reproduced from Vineis P et al. Tobacco and Cancer: Recent Epidemiological Evidence. JCNI 2004;96:99–106. With permission from Oxford University Press.
Screening is therefore not an alternative to smoking cessation, and Dr. Mulshine stressed that every effort should still be made to continue to help people to stop smoking. Using data from a cost-utility analysis of lung cancer screening based on data from the NLST, he showed that repeat annual screening in the high-risk population is predicted to be highly cost-effective and could save more than 985,000 quality-adjusted life years throughout the next 15 years. Adding a smoking cessation intervention to that screening process could further improve its cost-effectiveness [Villanti AC et al. PLoS One 2013].
The demographics of cancer are also evolving in general. The United States' population is aging, and during the next 20 years, the incidence of cancer will increase in older adults compared with younger adults. In 2030, the incidence of all cancers is predicted to increase by about 60% in older adults and by about 11% in younger adults [Smith B et al. J Clin Oncol 2009]. Consequently, the need for lung cancer screening is high.
TREATMENT GUIDELINES FOR NSCLC
Health care professionals have access to numerous published guidelines that serve as important resources to assist the clinical decision-making process. ESMO [Vansteenkiste I et al. Ann Oncol 2013; Fruh M et al. Ann Oncol 2013], ASCO [Pisters KMW et al. J Clin Oncol 2007], and NCCN [Ettinger DS et al. J Natl Compr Canc Netw 2013] all publish clinical practice guidelines for the management of lung cancer. Wilfried Eberhardt, MD, University Hospital Essen, Essen, Germany, compared these guidelines for early-stage and locally advanced NSCLC, demonstrating that despite their similarities, there are often subtle differences in their recommendations.
For example, in all 3 guidelines, the goal of surgery in stage I and II disease is complete resection. There are, however, some differences with respect to mediastinal evaluation. In the ESMO guidelines, mediastinal lymph node (LN) dissection is based on the International Association for the Study of Lung Cancer (IASCL) criteria. And although systematic LN dissection and sampling are discussed in the NCCN guidelines, no data are given in the ASCO guidelines for the surgical approaches to these cases.
For diagnosis of locally advanced stage III disease, both the ESMO and NCCN guidelines recommend the use of CT and positron emission tomography bronchoscopy. Similarly, they both advise endobronchial ultrasound and endoscopic ultrasound of suspicious LNs, followed by mediastinography if those investigations are negative. No data are available in the ASCO guidelines, however, to guide the diagnostic approach.
Enriqueta Felip, MD, PhD, Vall d'Hebron University Hospital, Barcelona, Spain, compared the guidelines for treatment of stage IV disease. For patients with epidermal growth factor receptor (EGFR)-mutated NSCLC, for example, the ESMO guidelines recommend the EGFR tyrosine kinase inhibitors (TKIs) erlotinib and gefitinib as the preferred first-line treatment, whereas the NCCN recommends erlotinib or afatinib. Prof. Felip also shared the recently published recommendations from the 2nd ESMO Consensus Conference on Lung Cancer, another source of recommendations for lung cancer management. These guidelines address questions on various areas, including treatment of advanced NSCLC, and they also recommend the use of an EGFR-TKI as the preferred first-line treatment for EGFR-mutated NSCLC [Besse B et al. Ann Oncol 2014].
In conclusion, Prof. Eberhardt emphasized that no single clinical practice guideline is optimal, but all are complementary. Because the guidelines range from purely evidence-based recommendations, as in the ASCO and ESMO publications, to very practical guidelines with algorithms that discuss clinical scenarios, as in the NCCN publication, he stressed that the choice of guideline must be governed by the physician's needs and expectations.
- © 2014 MD Conference Express®