Summary
This article presents results from a randomized clinical trial, demonstrating that self-administered lidocaine gel does not significantly reduce the pain associated with intrauterine device (IUD) insertion in nulliparous women, but does reduce pain during placement of the speculum and tenaculum, and therefore may be used prior to gynecological examinations and procedures [Rapkin RB et al. Obstet Gynecol 2014].
- Contraception Clinical Trials
- Contraception
- Obstetrics & Gynecology Clinical Trials
- Obstetrics & Gynecology
Rachel Becker Rapkin, MD, MPH, Morsani College of Medicine, University of South Florida, and University of Pittsburgh Medical Center, Pennsylvania, USA, presented results from a randomized clinical trial, demonstrating that self-administered lidocaine gel does not significantly reduce the pain associated with intrauterine device (IUD) insertion in nulliparous women, but does reduce pain during placement of the speculum and tenaculum, and therefore may be used prior to gynecological examinations and procedures [Rapkin RB et al. Obstet Gynecol 2014].
Despite their safety and efficacy in nulliparous women, IUDs remain underutilized, often because of the fear of pain associated with their insertion. To date, studies to investigate pain management have shown that the use of analgesics during IUD placement provide no clear benefit [Rapkin RB et al. Obstet Gynecol 2014]. Prof. Rapkin and colleagues conducted a randomized, double-blind, placebo-controlled trial to evaluate the efficacy of self-administered vaginal lidocaine gel before IUD insertion in nulliparous women with no history of pregnancy in the last 6 weeks. Exclusion criteria included women in whom there was prior IUD use or failed attempt at IUD insertion, use of narcotics or benzodiazepines within 24 hours, and contraindication to IUD use or amide anesthetic.
Women were randomized to self-administer 10 mL 2% lidocaine (n=30) or placebo gel (n=29) 5 minutes prior to IUD insertion. They used an electronic visual analog scale (VAS) to measure pain at baseline, during placement of the speculum and tenaculum, insertion of the IUD, and 5 minutes after speculum removal. Women were evaluated 1 week after IUD insertion to assess their need for pain medication after the procedure and their overall satisfaction.
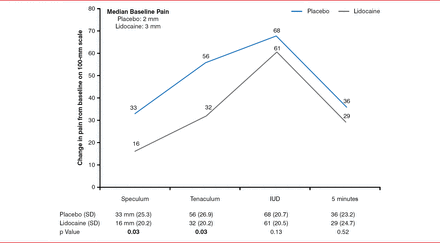
The median pain score (Figure 1) was not different between baseline and IUD insertion in the lidocaine and placebo groups (61 mm vs 68 mm; p=0.13), and 5 minutes after speculum removal (29 mm vs 36 mm; p=0.52); however, differences were significant at the time of speculum placement (16 mm vs 33 mm; p=0.03) and tenaculum placement (32 min vs 56 mm; p=0.03).
Median Pain Scores During the Intrauterine Device Insertion Procedure
Reproduced with permission from RB Rapkin, MD, MPH.
At the 1-week follow-up phone call, 84% of women in the lidocaine group and 90% of women in the placebo group were somewhat or very satisfied with their IUD placement, and 83% and 93%, respectively, would probably or definitely recommend the IUD to a friend.
Prof. Rap kin concluded that although self-administered vaginal lidocaine does not reduce pain with IUD insertion in nulliparous women, it does significantly decrease pain after speculum and tenaculum placement and therefore may be used prior to gynecological procedures involving these instruments.
- © 2014 MD Conference Express®