Summary
Results from the Efficacy and Safety of GTR in Comparison to Copaxone trial [GATE; NCT01489254] show that GTR, a generic form of glatiramer acetate (GA), is as effective and safe as the brand-name drug for the treatment of patients with relapsing-remitting multiple sclerosis (RRMS).
- Demyelinating Diseases
- Neurology Clinical Trials
- Demyelinating Diseases
- Neurology Clinical Trials
- Neurology
Results from the Efficacy and Safety of GTR in Comparison to Copaxone trial [GATE; NCT01489254] reported by Jeffrey A. Cohen, MD, Cleveland Clinic, Cleveland, Ohio, USA, show that GTR, a generic form of glatiramer acetate (GA), is as effective and safe as the brand-name drug for the treatment of patients with relapsing-remitting multiple sclerosis (RRMS).
Medications contribute significantly to the cost of care for patients with multiple sclerosis (MS). Generic versions of GA that may help reduce treatment costs are being evaluated, but neither the US Food and Drug Administration nor the European Medicines Agency has issued a final guidance for establishing bioequivalence for GA.
GATE is an ongoing multinational, parallel-group, equivalence study designed to demonstrate that GTR is as efficacious as GA in patients with RRMS. Men and women aged 18 to 55 years with RRMS according to the McDonald criteria (2010)—an Expanded Disability Status Scale (EDSS) score ≤ 5.5, ≥ 1 relapse in the year before screening, and 1 to 15 T1-gadolinium enhanced (Gd+) lesions on magnetic resonance imaging (MRI) at screening—were eligible to enroll. Participants were randomized to GTR (n = 353) or GA (n = 357) 20 mg, or placebo (n = 84), daily for 9 months, followed by 15 months of open-label treatment with GTR. Centrally analyzed MRI was performed at baseline and at months 7, 8, 9, 12, 18, and 24. Patients completed the EDSS at baseline and at months 6, 9, 12, 18 and 24. The primary study end point of the double-blind portion was the number of T1-Gd+ lesions at months 7 to 9 of treatment. Safety was assessed by adverse event (AE) reporting and tolerability, which was assessed at the initiation of therapy, and at months 3, 9, and 12. Dr Cohen reported the 9-month results.
At baseline, patients (mean age, approximately 33 years; approximately 67% women) had a mean disease duration of 5.5 to 6.4 years, a median of 2 Gd+ lesions (mean, 2.5–2.8), and a mean 1.9 relapses within the 2 years prior to study enrollment. More than 90% of participants completed the double-blind phase of the trial.
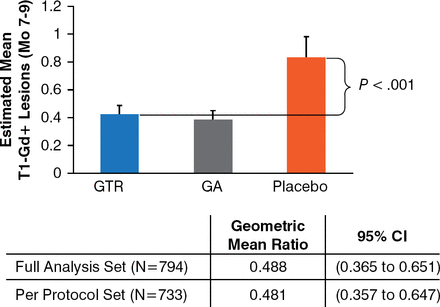
Both GTR and GA significantly reduced the number of T1-Gd+ lesions as compared with the placebo (P < .001; Figure 1).
Study Sensitivity: T1-Gd+ Lesions
GA, glatiramer acetate; GTR, a generic form of GA; T1-Gd+, T1-gadolinium enhanced.
Reproduced with permission from JA Cohen, MD.
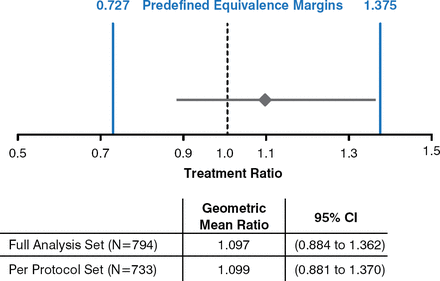
GTR was equivalent to GA in reducing the number of T1-Gd+ lesions based on the predefined equivalence margins (Figure 2).
Equivalence: T1-Gd+ Lesions
T1-Gd+, T1-gadolinium enhanced.
Reproduced with permission from JA Cohen, MD.
There were no differences between the 2 active treatments in other MRI end points at 9 months: change in T2 lesion number or volume (mm3), change in T1-hypo lesion volume (mm3), or change in brain volume (cm3). Clinical end points were similar between GTR and GA (Table 1).
Clinical End Points at Month 9
Overall safety and tolerability were excellent and similar for GTR and GA. The most common drug-related AEs were injection site reaction (16.4% of GTR-treated patients vs 17.1% of GA-treated patients) and immediate postinjection reaction (6.8% and 5.0%, GTR vs GA, respectively). The rate of serious or severe AEs was low. Drug-related serious AEs were reported by 3 (0.8%) GTR-treated patients and 4 (1.1%) GA-treated patients. No patients died. Local tolerability (pain, itchiness, redness, swelling, lumps) was similar between the GTR and GA groups.
Data from the open-label study is expected in 2015 and will include 2-year safety and efficacy data, data on switching (from GA to GTR), and immunogenicity data for both the core and open-label portions of the study.
- © 2014 MD Conference Express®