Summary
Treatment with the sphingosine 1-phosphate (S1P) receptor modulator, RPC1063, resulted in a substantial decrease in gadolinium-enhancing (Gd+) lesions and the number of new or enlarging T2 lesions in patients with relapsing-remitting multiple sclerosis (RRMS). This article presents data from the Efficacy and Safety Study of RPC1063 in Relapsing Multiple Sclerosis Patients trial [Radiance Study; NCT01628393].
- Demyelinating Diseases
- Neurology Clinical Trials
- Demyelinating Diseases
- Neurology Clinical Trials
- Neurology
Treatment with the sphingosine 1-phosphate (S1P) receptor modulator, RPC1063, resulted in a substantial decrease in gadolinium-enhancing (Gd+) lesions and the number of new or enlarging T2 lesions in patients with relapsing-remitting multiple sclerosis (RRMS). Amit Bar-Or, MD, Montreal Neurological Institute, Montreal, Ontario, Canada, presented data from the Efficacy and Safety Study of RPC1063 in Relapsing Multiple Sclerosis Patients trial [Radiance Study; NCT01628393].
Targeting the S1P receptor family is an approach that has been explored as a treatment for patients with RRMS [Halmer R, Walter S, Fasbender K. Cell Physiol Biochem. 2014. RPC1063 is a novel agent that modulates S1P receptors 1 and 5, and has been previously evaluated in healthy subjects. The purpose of the Radiance Study was to further evaluate RPC1063 in patients with RRMS.
In the phase 2 Radiance Study, 258 adult patients with RRMS were randomly assigned to receive 24 weeks of 0.5 mg or 1 mg of RPC1063, or placebo. Upon completion of the treatment period, a blinded extension study was conducted in which patients who received RPC1063 continued treatment, and patients who received placebo were assigned to 0.5 mg or 1 mg of RPC1063. Patients aged 18 to 55 were eligible to enroll in the study if they had an Expanded Disability Status Scale (EDSS) score of 0 to 5.0 at baseline and met ≥ 1 of the RRMS criteria, which included ≥ 1 documented relapse within the prior 12 months, or ≥ 1 documented relapse plus ≥ 1 Gd+ lesion(s) within the previous 24 months.
The primary end point of the Radiance Study was the cumulative number of Gd+ lesions. Secondary end points included the number of Gd+ lesions at week 24, the number of cumulative or enlarging T2 lesions, and the annualized relapse rate (ARR). At baseline, the mean EDSS score ranged from 2.85 to 2.94, the mean number of relapses over 12 months from 1.3 to 1.5, and the number of Gd+ lesions from 0.9 to 1.4.
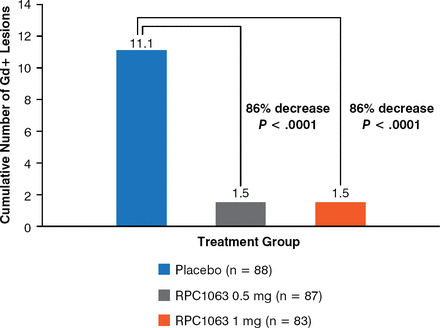
The cumulative number of Gd+ lesions significantly decreased by 86% (P < .0001) in patients who received either dose of RPC1063 as compared with patients who received the placebo from week 12 to 24 (Figure 1). In addition, at week 24, the mean number of Gd+ lesions significantly decreased by 91% and 94% (P < .0001 for both) in patients who received 0.5 mg and 1 mg of RPC1063, respectively, as compared with patients who received the placebo. Similarly, the cumulative number of new or enlarging T2 lesions significantly decreased by 84% and 91% (P < .0001 for both) in the 0.5 mg and 1 mg RPC1063 arms as compared with the placebo arm from week 12 to week 24. There was a dose-dependent trend toward a decrease in ARR, with a rate of 0.5 in the placebo arm, 0.35 in the 0.5-mg RPC1063 arm, and 0.24 in the 1-mg RPC1063 arm.
Effect of RPC1063 on Total Number of Gd+ Lesions Over 12 Weeks
Gd+, gadolinium-enhancing.
Reproduced with permission from JA Cohen, MD.
During the core, 24-week portion of the Radiance Study, the number of patients who experienced ≥ 1 treatment-emergent adverse event (TEAE) was 59.1% in the placebo arm, and 56% and 47% in the 0.5-mg and 1-mg RPC1063 arms, respectively. The most common TEAEs in patients who received RPC1063 included nasopharyngitis, headache, and urinary tract infection. During the study, 3 serious TEAEs were reported but deemed unrelated to the study drug. However, there were 3 cases of elevated alanine aminotransferase (≥ 3 times the upper level of normal), but without associated clinical signs. There were no cases of discontinuation due to an AE.
In conclusion, Dr Cohen indicated that treatment of patients with RRMS with RPC1063 resulted in substantial reductions in magnetic resonance imaging measures and disease activity, with an overall good safety profile found with this phase 2 Radiance Study. A phase 3 portion of the Radiance Study [NCT02047734] is ongoing and a phase 3 SUNBEAM trial, evaluating RPC1063 vs interferon beta-1a, is in the planning phase.
- © 2014 MD Conference Express®