Summary
Precision medicine uses traditional and emerging concepts of the genetic and environmental basis of disease to individualize prevention, diagnosis and treatment [National Cancer Institute, 2011]. It integrates the tumor genetics of individual patients into medical practice. The potential value of the targeted and precise approach is great. This article discusses advances in precision medicine from the perspective of prospective clinical trials. A specific example of melanoma as a model for personalized cancer medicine is also presented.
- Oncology Genomics
- Oncology
- Oncology Genomics
Apostolia M. Tsimberidou, MD, PhD, University of Texas MD Anderson Cancer Center, Houston, Texas, USA, discussed advances in precision medicine from the perspective of prospective clinical trials. Precision medicine uses traditional and emerging concepts of the genetic and environmental basis of disease to individualize prevention, diagnosis and treatment [National Cancer Institute, 2011]. It integrates the tumor genetics of individual patients into medical practice. The potential value of the targeted and precise approach is great. For instance, in a multicenter study in patients with metastatic lung adenocarcinoma, the median survival was longer in patients with an oncogenic driver and genotype-directed therapy compared with those with any oncogenic driver who did not receive genotype-directed therapy [Kris MG et al. JAMA 2014].
Current challenges to precision medicine include incomplete validation of the plethora of tests that are constantly evolving, because the patients with specific targetable alterations treated with targeted agents are too few to analyze, and limited access to targeted agents. Prospective randomized trials with innovative designs that explore different types of genetic profiling and modeling hold the potential of permitting the assessment of the effectiveness of molecular profiling, which will enable the selection and use of the optimal targeted therapy.
ONGOING CLINICAL TRIALS WITH TARGETED THERAPY BASED ON TUMOR TYPE
The Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination study [BATTLE 2; NCT01248247] is a randomized Phase 2 trial with patients with advanced, refractory non–small cell lung cancer (NSCLC). The primary objective is 8-week disease control rate; 400 patients are to be randomly assigned based on the results of patient tumor profiling. To date, results from 167 patients have demonstrated an improved disease control rate with the combined use of AKT and MEK inhibitors [Papadimitrakopoulou VA et al. ASCO 2014].
The Intergroup Trial UNICANCER UC 0105–1305/IFCT 1301: Efficacy of Targeted Drugs Guided by Genomic Profiles in Metastatic NSCLC Patients, an Intergroup study assessing the efficacy of targeted drugs guided by genomic profiles in patients with metastatic NSCLC [SAFIR 02 – Lung trial; NCT02117167] is a multicenter, randomized Phase 2 trial that uses high-throughput genome analysis to guide treatment based on identified tumor aberrations.
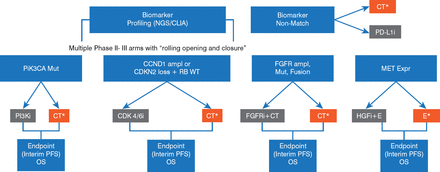
The Lung-MAP: S1400 Biomarker-Targeted Second-Line Therapy in Treating Patients With Recurrent Stage IIIB-IV Non–Small Cell Lung Cancer study [Lung-MAP (SWOG 1400); NCT02154490] is a Phase 2/3 screening/clinical registration “master protocol” that will molecularly profile patients with advanced NSCLC. Patients with genetic alterations will be randomly assigned to investigational targeted therapy versus standard therapy (Figure 1).
Schema of the Lung-MAP: S1400 Biomarker-Targeted Second-Line Therapy in Treating Patients With Recurrent Stage IIIB-IV Non–Small Cell Lung Cancer Trial
CT=chemotherapy (docetaxel or gemcitabine); E=erlotinib; TT=targeted therapy.
Reproduced with permission from A. M. Tsimberidou, MD, PhD.
The Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging and Molecular Analysis 2 trial [I-SPY TRIAL 2; NCT01042379] is a multicenter, Phase 2, neoadjuvant trial for women with high-risk stage II or III breast cancer. This study uses adaptive randomization within biomarker subtypes to evaluate novel agents added to standard chemotherapy. The primary endpoint is pathologic complete response. In 2013, the investigators announced the graduation of 2 regimens for 2 different biomarker signatures: for patients with triple-negative breast cancer when added to standard, presurgery chemotherapy, veliparib combined with carboplatin improved the response rate. Neratinib also demonstrated a high probability for success in a Phase 3 study in patients with HER2-positive, hormone receptor–negative disease.
ONGOING CLINICAL TRIALS WITH TARGETED THERAPY ACROSS TUMOR TYPES
In an international study to select rational therapeutics on the basis of the analysis of matched tumor and normal biopsies in subjects with advanced malignancies (A Study to Select Rational Therapeutics Based on the Analysis of Matched Tumor and Normal Biopsies in Subjects With Advanced Malignancies [WINTHER; NCT01856296]), tumor tissue will be used for molecular profiling and tumor and normal tissue for functional genomic microarrays and gene expression profiling. Patients with targetable molecular alterations will be treated with targeted therapy, and the remaining patients will be treated using a predetermined predictive algorithm of efficacy of drugs based on functional genomic analysis.
The Randomized Phase 2 Trial Comparing Therapy Based on Tumor Molecular Profiling Versus Conventional Therapy in Patients With Refractory Cancer trial [SHIVA; NCT0177458] has almost completed patient accrual.
The NCI Molecular Profiling-Based Assignment of Cancer Therapy Study for Patients With Advanced Solid Tumors [MPACT; NCT01827384] is designed to assess the utility of genome sequencing to determine therapy and improve patient outcomes in early-phase trials independent of tumor histology.
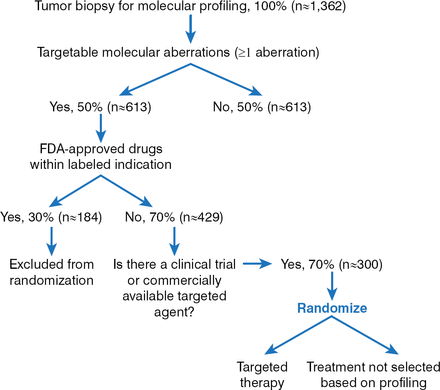
The Randomized Study Evaluating Molecular Profiling and Targeted Agents in Metastatic Cancer 2 trial [IMPACT; NCT02152254] is a large-scale, randomized Phase 2 study evaluating molecular profiling and targeted therapy in metastatic cancer. The objective of the study is to determine whether patients treated with a targeted therapy selected based on tumor molecular analysis have longer PFS from the time of randomization than do those whose treatment is not selected based on molecular analysis (Figure 2).
Schema of the IMPACT 2 Trial
Reproduced with permission from A. M. Tsimberidou, MD, PhD.
These and future prospective trials should speed the identification of drugs that are specifically effective for the target tumors.
Alexander M. Eggermont, MD, PhD, Gustave Roussy Comprehensive Cancer Center, Paris, France, discussed melanoma as a model for personalized cancer medicine. The presentation focused on 2 main aspects of personalized cancer medicine: mutation-driven drug development and the use of innovative immunomodulation strategies.
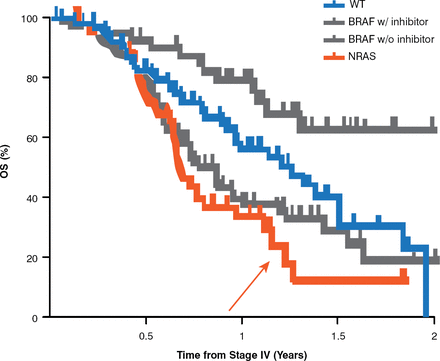
The genesis of mutation-driven drug development was a study almost a decade old that chronicled the different oncogenic mutations profiles in primary melanomas, which identified the potential selective advantage of the tyrosine kinase inhibitor imatinib in this setting [Curtin JA. J Clin Oncol 2006]. The influence of patient age on the changing pattern of prevalent mutations has since been documented [Rubenstein JC et al. J Transl Med 2010], as has the nature of the mutation and the treatment offered (Figure 3) [Jakob JA et al. Cancer 2012].
Overall Survival of Wild-Type Versus BRAF With and Without Treatment With Inhibitor and NRAS
OS=overall survival; WT=wild-type.
Reproduced with permission from John Wiley & Sons from Jakob JA, Bassett RL Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012;118(16):4014–4023.
Still, the improvements documented in PFS in therapies to BRAF mutations may be more transient than long lasting [McArthur GA et al. Lancet Oncol 2014]. Whether this is the case with other mutation-targeted therapies requires study. Furthermore, contrary to expectations, targeted therapy is not free from adverse effects. Toxicity is always major consideration in the adjuvant setting, which includes even precisely targeted therapies. Dr. Eggermont cautioned that one needs to remember that tumors are not a static target; they can change with time, acquiring resistance to the treatment agent through mutations that can change tumor physiology, which in turn influences treatment efficacy.
Innovative immunomodulation is being increasingly used in targeted therapy. The central tenet of immunomodulation is breaking tolerance using immune checkpoint blockers. In this approach, targeting an inhibitor of the checkpoint blockers is more important and fundamentally useful than stimulation of activators. For example, blocking antibodies to T cell surface receptors, including CTLA-4, PD-1, TIM-3, and LAG-3, can blunt cell stimulation. This receptor blockade can occur naturally when T cells interact with tumor cells. In essence, the tumor cells stifle T cell action. But blockade of the T cell receptors prior to tumor cell interaction can help preserve the antitumor activity. The anti-CTLA-4 monoclonal antibody ipilimumab was the first therapy to improve overall survival (OS) in unresectable or metastatic melanoma [Hodi FS et al. N Engl J Med 2010]. The benefit was not instantaneous but rather required about 3 months to manifest. Thereafter, OS was significantly better than treatment with glycoprotein 100 peptide vaccine. Furthermore, blockade of PD-1 can also obviate interaction of the T cell surface protein with tumor cells receptor, which otherwise blunts T cell function. By blocking the inhibitor, the immune response against tumors can be bolstered.
Targeted therapy is best envisioned in concert with radiation therapy and chemotherapy as a multipronged treatment strategy. This approach is in its infancy.
- © 2014 MD Conference Express®