Summary
Aspirin may be an effective adjuvant treatment for patients with cancer; this has been suggested by long-term follow-up of randomized trials that were originally designed to determine the vascular effects of aspirin. This article discusses Add-Aspirin, a Phase 3 double-blind multicenter trial that will determine whether taking low-dose aspirin on a regular basis will improve the prognosis of patients who have recently undergone primary curative treatment for nonmetastatic colorectal, gastroesophageal, breast, or prostate cancer.
- Oncology Clinical Trials
- Gastrointestinal Cancers
- Oncology Clinical Trials
- Gastrointestinal Cancers
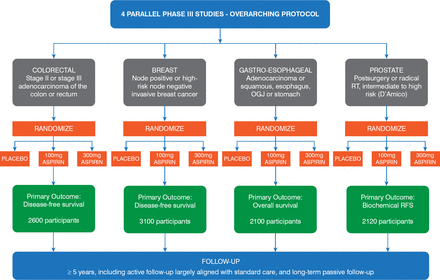
Aspirin may be an effective adjuvant treatment for patients with cancer; this has been suggested by long-term follow-up of randomized trials that were originally designed to determine the vascular effects of aspirin. Low-dose aspirin, taken daily for 5 years, was associated with a decreased risk of cancer, particularly that of gastrointestinal tumors [Rothwell PM et al. Lancet. 2011]. Aspirin may also reduce the risk of developing BRAF wild-type colorectal cancer [Nishihara R et al. JAMA. 2013]. Other studies have suggested that mutations in the PIK3CA gene (phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit alpha) may identify patients with colorectal cancer who would derive even greater benefit from adjuvant aspirin therapy [Liao X et al. N Engl J Med. 2012; Domingo E et al. J Clin Oncol. 2013; Kothari N et al. J Clin Oncol. 2014]. Another study showed no benefit from aspirin in patients with wild-type PIK3CA, although the study was inadequately powered [Reimers MS et al. JAMA Intern Med. 2014]. Therefore, the hypothesis that aspirin might improve prognosis in patients with colorectal cancer with PIK3CA mutations requires prospective evaluation in randomized trials. Add-Aspirin is a Phase 3 double-blind multicenter trial that will determine whether taking low-dose aspirin on a regular basis will improve the prognosis of patients who have recently undergone primary curative treatment for nonmetastatic colorectal, gastroesophageal, breast, or prostate cancer. Ruth E. Langley, MD, Medical Research Council Clinical Trials Unit at University College London, London, United Kingdom, described the Add-Aspirin trial in the presentation “Are the Benefits of Aspirin in Colorectal Cancer Limited to PIK3CA Mutated Cancers?” Add-Aspirin provides an opportunity to investigate the potential association between PIK3CA mutations and benefit from aspirin after a colorectal cancer diagnosis. The Add-Aspirin study plan is presented in Figure 1.
Add-Aspirin Study Plan
OGJ=esophagogastric junction; RFS=relapse-free survival; RT=radiation therapy
Reproduced with permission from R Langley, MD.
The Add-Aspirin study is aiming to register approximately 11,000 potential participants and randomize 9920, including 2600 patients with colorectal cancer. The study design includes an active run-in period prior to randomization during which participants take 100 mg of aspirin daily for about 8 weeks. During the run-in period, the toxicity of and adherence to the aspirin therapy will be determined. If funding is available, the PIK3CA mutation status in patients with colorectal cancer will be determined and used as a stratification factor as part of the random assignment to treatment. After the run-in period, patients will be randomly assigned within each tumor-type cohort, 1:1:1, to aspirin–100 mg daily, aspirin–300 mg daily, or matching placebo for at least 5 years. Patients aged at least 75 years will be randomly assigned 2:1 to 100 mg of aspirin or placebo. Each tumor-specific cohort is individually powered with separate co—primary outcome measures, incorporating overall survival alongside a tumor-specific measure.
For patients in the colorectal cohort, there is a planned subgroup analysis for disease-free survival (DFS) in the PIK3CA-mutated group (approximately n = 390 or 15% of randomized patients). This analysis is powered at 90% to detect a hazard ratio of .5 in favor of aspirin versus placebo in DFS.
Add-Aspirin is designed to determine if long-term low-dose aspirin therapy will improve prognosis in patients with successfully treated colorectal, gastroesophageal, breast, or prostate cancer. Different signaling pathways may influence the development of primary tumors and the development and spread of tumor metastases. Add-Aspirin may determine if aspirin can inhibit one or more of these pathways. Add-Aspirin also offers the opportunity to address the role of PIK3CA mutation status in the colorectal patient cohort and determine whether the benefits of aspirin after a colorectal cancer diagnosis are influenced by PIK3CA mutation status.
- © 2014 MD Conference Express®