Summary
Global cancer prevention experts, gastrointestinal cancer physicians, and researchers attended the International Society of Cancer Prevention consensus conference to discuss the current science of colorectal cancer (CRC) carcinogenesis, screening, and preventive intervention; to develop a consensus statement encompassing current recommendations for CRC screening, genetic assessment, and cancer prevention interventions; and to further delineate knowledge gaps that need to be addressed in future CRC prevention research. This article presents the highlights from the CRC prevention consensus meeting.
- Gastrointestinal Cancers
- Gastrointestinal Cancers
Global cancer prevention experts, gastrointestinal cancer physicians, and researchers attended the International Society of Cancer Prevention (ISCaP) consensus conference to discuss the current science of colorectal cancer (CRC) carcinogenesis, screening, and preventive intervention; to develop a consensus statement encompassing current recommendations for CRC screening, genetic assessment, and cancer prevention interventions; and to further delineate knowledge gaps that need to be addressed in future CRC prevention research. Powel H. Brown, MD, PhD, University of Texas MD Anderson Cancer Center, Houston, Texas, USA, presented the highlights from the CRC prevention consensus meeting.
All participating countries recommend screening for CRC, but the methods used vary by country. “It was striking how different these colorectal screening recommendations were,” commented Dr Brown. While colonoscopy is widely used in the United States, other countries use the fecal occult blood test (FOBT), the fecal immunochemical test (FIT), or sigmoidoscopy. Even though the screening methodologies differ, all experts agreed that the current screening compliance level of 40% to 60% is far too low. Of all types of cancer screening, CRC screening may have the highest impact to prevent disease and affect mortality. The panel therefore believes that some type of CRC screening should be strongly recommended, usually after age 50 years, and that the promotion of screening compliance should be a priority.
The largest variables in colonoscopies are patient and operator dependent, as bowel preparation and the experience of the endoscopist influence the success of the procedure. Novel colonoscopy techniques are in development and may help reduce this variability and increase patient compliance: Water exchange colonoscopy provides better visualization of the colon and may require little or no sedation. Capsule colonoscopy is still in development but is a promising technique that may lead to better screening acceptance.
The next generation of fecal and blood tests are also being developed. The Cologuard test is a multitarget DNA test with an FIT based on exfoliated DNA in the feces. This noninvasive method requires no bowel preparation and no diet or medication restrictions; it can be performed at home and mailed; it is affordable; and it provides results quickly. The DeeP-C trial [Imperiale TF et al. N Engl J Med. 2014] was a prospective study of ∼ 10,000 asymptomatic patients aged 50 to 84 years. Samples were tested by both Cologuard and an FIT alone. Cologuard had better sensitivity and specificity when compared with the FIT. Blood-based tests for cancer-specific DNA (Epi ProColon) and microRNA assays also appear promising. Despite these advances, for now, the FOBT, the FIT, colonoscopy, and sigmoidoscopy remain the standard recommendations for CRC screening.
The panel also agreed that the prevention interventions of diet, healthy weight, and exercise continue to be effective prevention strategies. The current recommendation from the American Heart Association and other organizations is 30 to 45 minutes of vigorous exercise 3 to 5 times per week, and there is strong evidence that supports this recommendation. “It is also important to point out that less aggressive exercise, as much as just 15 minutes several days a week, [shows] a detectable improvement in cancer incidence,” noted Dr Brown. While epidemiologic evidence indicates that diet affects risk, this has not been confirmed in randomized clinical trials testing different dietary strategies. Despite this, a diet lower in red meat and higher in fruits, vegetables, and fiber is recommended. New e-health techniques, such as fitness-tracking wristbands and smartphone apps that measure physical activity, may complement behavioral interventions.
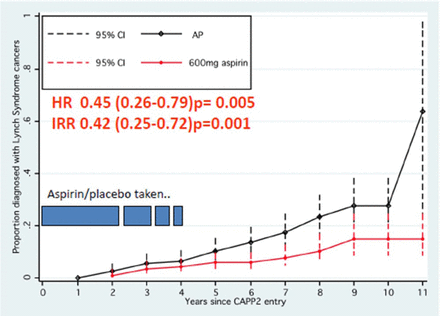
The conference also reviewed chemoprevention strategies for CRC. There is strong evidence suggesting that aspirin lowers cancer incidence and cancer-related mortality [Rothwell PM et al. Lancet. 2012; 2011; 2010]. The effect of aspirin on the incidence of CRC appears to be delayed and is typically seen 5 years after aspirin therapy is started [Cook NR et al. Ann Int Med. 2013; Rothwell PM et al. Lancet. 2011]. The reason for this is unknown, but it is possible that precancerous cells are destroyed before developing into a detectable cancer. The CAPP2 study [Burn J et al. N Engl J Med. 2008] was conducted in patients with Lynch syndrome, an inherited type of colon cancer. The original publication from this study reported no effect of aspirin (600 mg/d) out to 4 years. However, extended data from the CAPP2 study presented at the ISCaP meeting showed that a treatment effect is evident with > 5 years of aspirin therapy (Figure 1).
CAPP2 Per-Protocol Analysis: All Lynch Syndrome Cancers
AP=aspirin placebo.
Burn et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;9809:2081–2087. Republished with permission from Lancet Publishing Group; permission conveyed through Copyright Clearance Center, Inc.
Researchers have not yet determined the optimal dose of aspirin, but the available data suggest a benefit at doses ≤ 100 mg/d. The risk of gastrointestinal bleed is age dependent and is slight in patients aged 50 to 65 years. The use of aspirin is therefore recommended for CRC prevention, particularly in patients with a family history of CRC. Daily doses of 81 to 100 mg are considered safe and effective, but it is possible that higher doses may be even more effective. The CAPP3 study is being designed to test this hypothesis. In this study, patients with Lynch syndrome will be randomized to blinded doses of aspirin (100, 300, or 600 mg/d) for several years and then switched to open-label aspirin (100 mg/d).
Other agents are being investigated as potential chemopreventive agents in CRC. The use of cyclooxygenase-2 inhibitors is limited by their cardiac side effects, but investigations are ongoing into other ways to target the same inflammatory pathway. These include the use of EP antagonists, CXCR2 antagonists, and downstream pathways such as peroxisome proliferator-activated receptors. Difluoromethylornithine and sulindac may help young patients with familial adenomatous polyposis before having colectomy. In addition, preclinical data suggest that peptide vaccines may be effective in the treatment of patients with mismatch DNA-repair defects.
In his concluding remarks, Dr Brown reemphasized the importance of CRC screening. “Increased uptake of screening was considered the most important aspect of colon cancer prevention by the entire panel and the most cost effective.”
- © 2014 MD Conference Express®