Summary
Sodium-glucose cotransporter 2 (SGLT2) inhibitors (are novel agents, and this article discusses how these agents can be used in the treatment of patients with diabetes.
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
Sodium-glucose cotransporter 2 (SGLT2) inhibitors (SGLT2i) are novel agents, and there was a special session at the European Association for the Study of Diabetes (EASD) 2014 annual meeting on how these agents can be used in the treatment of patients with diabetes.
HOW THESE AGENTS WORK
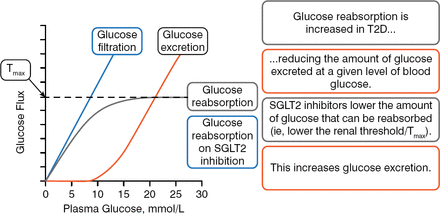
Anna Solini, MD, PhD, University of Pisa, Pisa, Italy, opened the session to explain how this class of agents works at the molecular level. SGLT2 is a low-affinity, high-capacity glucose transporter, located in the S1 and S2 segments of the renal proximal tubules, that accounts for around 90% of renal glucose reabsorption (RGR) in healthy individuals. SGLT1 is a low-capacity, high-affinity glucose transporter that accounts for about 10% of RGR in the more distal S3 segment of the tubular lumen. These 2 separate transporters function together such that there should be no urinary glucose excretion (UGE) when the concentration of glucose is 180 to 190 mg/dL in healthy individuals. At levels higher than this concentration, urinary glucose excretion occurs. Patients with type 2 diabetes mellitus (T2DM) have lower amounts of urinary glucose excretion for any given level of blood glucose (BG). In Farber's seminal 1951 work, he showed that patients with T2DM had an elevated capacity for renal glucose reabsorption.
SGLT2i lower the amount of glucose that can be reabsorbed and increase the amount of urinary glucose excretion (Figure 1).
Renal Glucose Reabsorption and SGLT2 Inhibition
SGLT2, sodium glucose cotransporter 2; T2D, type 2 diabetes; Tmax, maximal renal tubule glucose reabsorption capacity.
Reproduced with permission from A Solini, MD, PhD.
In patients with T2DM, treatment with SGLT2i improved β-cell function and insulin sensitivity, despite the small reduction in insulin release and tissue glucose disposal, and the modest rise in endogenous glucose production, probably as a result of decreasing glucose toxicity [Merovci A. JCI 2014; Polidori D et al. Diabetes Care. 2013]. Postprandial hyperglycemia was improved with the use of less selective SGLT2 inhibition [Polidori D et al. Diabetes Care. 2013].
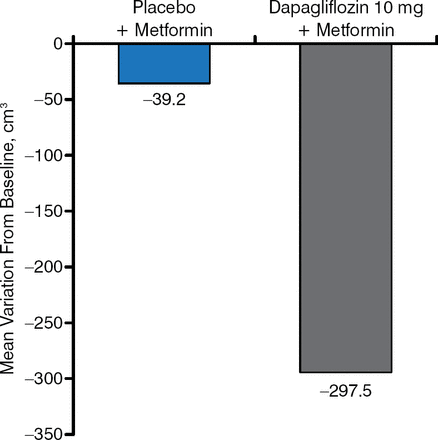
SGLT2i are consistently associated with weight loss. This weight loss does not appear to be related only to the loss of glucose, and it appears to be the result of a change in body composition [Cefalu WT et al. Lancet. 2013] and a fat mass reduction [Bolinder J et al. J Clin Endocrinol Metab. 2012] (Figure 2).
Change in Visceral Adipose Tissue as a Result of SGLT2 Inhibition
SGLT2, sodium glucose cotransporter 2.
Reproduced with permission from A Solini, MD, PhD.
Mechanisms involved in the reduction in blood pressure (BP) seen with this agent are still being investigated. Beyond a small osmotic diuresis and a change in plasma volume seen acutely, there also appears to be a thiazide-like effect on the renin-angiotensin aldosterone system [Lambers Heerspink HJ et al. Diabetes Obes Metab. 2013].
SGLT2i also have been reported to lower serum uric acid levels. The mechanism is unknown, but it has been hypothesized to be an interaction between blood glucose and uric acid through the uric acid transporter SLCTA9, also known as GLUT9, which can exchange glucose for uric acid [Chino Y et al. Biopharm Drug Dispos. 2014].
Prof Solini concluded by saying that SGLT2i are “smart, insulin-independent drugs” that also work by reversal of glucotoxicity with all related consequences, with efficacy related to the degree of renal function. She noted that there is still more to learn about selective versus less selective inhibition of SGLT2i.
CHANGING THE LANDSCAPE
Apostolos Tsapas, MD, PhD, Aristotle University, Thessaloniki, Greece, reviewed the extensive body of clinical, safety, and pharmacoeconomic data on SGLT2i. Table 1 shows SGLT2i with marketing authorization.
SGLT2 Inhibitors With Marketing Authorization
Prof Tsapas presented a comprehensive literature review of 2 meta-analyses [Liakos A et al. Diabetes Obes Metab. 2014; Vasilakou D et al. Ann Intern Med. 2013] and an updated search of electronic databases through June 30, 2014. It included 55 studies vs placebo (about 15000 patients) and 15 trials vs active agents (about 7000 patients) evaluating the efficacy and short-term safety of 7 available and investigational SGLT2i.
Overall findings from placebo-controlled trials showed that HbA1c reductions with SGLT2i were approximately −0.69% in all trials. Reductions in HbA1c were greater in trials in which the SGLT2i were tested as monotherapy (−0.80%) and less when used as add-on treatment (−0.63%). Based on their glucose-dependent mechanism of action, hypoglycemia risks were low unless used in combination with secretagogues. SGLT2i resulted in approximately −1.7 to −2.0 kg of weight loss. A slightly increased incidence of urinary tract infections (UTIs) with some SGLT2i were observed compared with placebo. A fourfold risk increase in incidence of genital tract infections (GTIs) compared with placebo was also observed. Minor elevations in low-density lipoprotein cholesterol (LDL-C) and total cholesterol were consistently observed with all SGLT2i along with an increase in high-density lipoprotein cholesterol (HDL-C), whereas triglyceride levels were improved. A small decline in renal function was noted with SGLT2i that tended to improve throughout time. Volume-related adverse events were more common among elderly patients and those patients with moderate renal impairment or treated with diuretics. An increased risk of fractures with dapagliflozin was noted in a dedicated trial that included patients with moderate renal impairment [Kohan DE et al. Kidney Int. 2014].
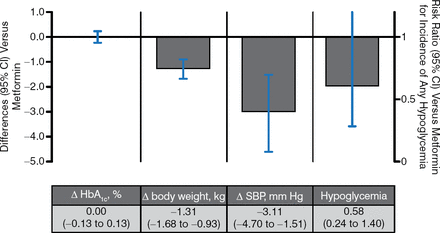
There were 6 trials of SGLT2i as monotherapy vs metformin (Figure 3). Reductions in blood glucose and the risk of hypoglycemia were similar regardless of how these agents were used. SGLT2i showed a greater weight loss effect with greater effects in BP lowering.
SGLT2 Inhibitors vs Metformin as Monotherapy
Δ, mean change from baseline vs metformin; SBP, systolic blood pressure; SGLT2, sodium glucose cotransporter 2.
Pooled results for 6 studies of SGLT2 inhibitors with ≥ 12 wk duration from published and gray literature sources through June 30, 2014 (search strategy adapted from Vasilakou et al. Ann Intern Med. 2013;159(4):262–274). Results are presented for the group allocated to the highest, most common dose among studies.
Reproduced with permission from A Tsapas, MD, PhD.
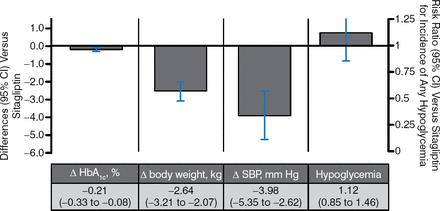
There has been one head-to-head monotherapy trial of an SGLT2i vs active therapy, empagliflozin vs sitagliptin, in which the SGLT2i and the comparator performed similarly in terms of glucose-lowering and hypoglycemia events. Empagliflozin induced greater reductions in body weight and BP; nevertheless, GTIs were more common in empagliflozin-treated patients (4% vs 1% for sitagliptin) [Roden M et al. Lancet Diabetes Endocrinol. 2013].
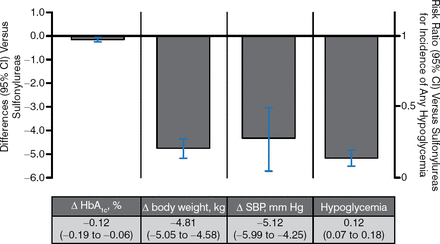
There have been 3 trials of SGLT2i vs sulfonylureas as an add-on to metformin, and these studies found less hypoglycemia and comparable glucose-lowering ability with SGLT2i (Figure 4).
SGLT2 Inhibitors vs Sulfonylureas as Add-on Therapy to Metformin
Δ, mean change from baseline vs metformin; SBP, systolic blood pressure; SGLT2, sodium glucose contransporter 2.
Pooled results for 3 trials of SGLT2 inhibitors with ≥ 12 wk duration from published and gray literature sources through June 30, 2014 (search strategy adapted from Vasilakou et al. Ann Intern Med. 2013;159(4):262–274). Results are presented for the group allocated to the highest, most common dose among studies.
Reproduced with permission from A Tsapas, MD, PhD.
In 5 trials of SGLT2i vs sitagliptin as an add-on to metformin, HbA1c-lowering and hypoglycemia rates were similar between SGLT2i and metformin. SGLT2i had more favorable effects on body weight and systolic blood pressure (Figure 5).
SGLT2 Inhibitors vs Sitagliptin as Add-on Therapy to Metformin
Δ, mean change from baseline vs metformin; SBP, systolic blood pressure; SGLT2, sodium glucose contransporter 2.
Pooled results for 5 trials of SGLT2 inhibitors with ≥ 12 wk duration from published and gray literature sources through June 30, 2014 (search strategy adapted from Vasilakou et al. Ann Intern Med. 2013;159(4):262–274). Results are presented for the group allocated to the highest, most common dose among studies.
Reproduced with permission from A Tsapas, MD, PhD
SGLT2i have also been studied in triple combination therapy (oral agents as well as insulin therapy) with good results.
The efficacy of SGLT2i declines with worsening renal function. Dapagliflozin should not be initiated in patients with estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2. Canagliflozin and empagliflozin should be discontinued in patients whose eGFR is < 45 mL/min/1.73 m2, and dose adjustments are required for patients with stage 3A chronic kidney disease based on local prescribing information. Dapagliflozin has reduced efficacy in patients with reductions in renal function and should be discontinued if the GFR is persistently < 60 mL/min/1.73 m2. Canagliflozin also has reduced efficacy in older patients.
Based on its insulin-independent mechanism, empagliflozin 25 mg has been preliminarily studied in patients with type 1 diabetes mellitus. An open-label, single-arm study reported findings of reduced HbA1c, body weight, total daily insulin dose, hypoglycemia, and fasting glucose [Perkins BA et al. Diabetes Care. 2014].
Meta-analyses on the cardiovascular safety of SGLT2i submitted to regulatory bodies showed a favorable profile for SGLT2i, with the exceptions of a hazard ratio for the nonfatal stroke risk ratio for canagliflozin (HR, 1.46; 95% CI, 0.83 to 2.58) and an imbalance in incidence of cardiovascular events during the first 30 days of the CANVAS trial (HR, 6.50; 95% CI, 0.85 to 49.66), possibly due to volume depletion [FDA Briefing Document, NDA 204042, 2013].
Ten cases of bladder cancer were reported in studies with dapagliflozin compared with 1 in the comparator group. The majority of these cases were detected due to hematuria. Current European guidelines do not recommend dapagliflozin to be given with pioglitazone. More long-term data are needed to better understand the relationship between SGLT2i and bladder cancer.
Prof Tsapas concluded that SGLT2i have well-established efficacy as antihyperglycemic agents (approximately −0.7% HbA1c reduction). These agents do not increase the risk for hypoglycemia and are associated with weight loss and mild reduction in BP. Based on their β-cell independent mechanism of action, SGLT2i can be used at any stage of diabetes and combined with any glucose-lowering agent, including insulin. SGLT2i may be a cost-effective option based on local priorities. Effects on long-term outcomes and diabetic complications remain to be determined. Special caution is warranted for patients at risk for volume depletion (ie, the elderly, those on diuretics, and those with renal impairment). The most common adverse events are UTIs and GTIs.
MANAGING UTIS
Jack D. Sobel, MD, Wayne State University, Detroit, Michigan, USA, carried on this discussion. Patients with diabetes, especially those with poorly controlled glycemia, are prone to developing genital mycotic infections—vulvovaginal candidiasis in women and Candida balanitis in men (almost exclusively in uncircumcised men) [Grandy S et al. Curr Ther Res Clin Exp. 2013].
Recommendations for treating women with conventional antimycotics are to avoid treating asymptomatic colonization, avoid short-term therapy, and consider treating sexual partners.
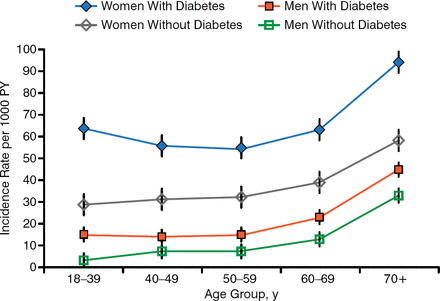
Elevated blood glucose and HbA1c are common in patients with glucose in the urine. The pathophysiology and treatment of UTIs in patients with diabetes are similar to those without, but diabetes increases the risk of UTIs (Figure 6) [Hirji J et al. Diabetes Complications. 2012]. People with diabetes are more likely to have asymptomatic bacteriuria (ASB), but this is not a problem and should not be treated except in pregnancy or prior to invasive procedures.
Incidence of UTI in Adults With T2DM
PY, person-year; T2DM, type 2 diabetes mellitus; UTI, urinary tract infection.
Reprinted from Journal of Diabetes and Its Complications, Vol. 26, Hirji I et al. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD). Pages 513–516, Copyright 2012, with permission from Elsevier.
The frequency and severity of UTIs are increased in patients with T2DM. A prior study found that 8.2% of patients with diabetes had ≥ 1 UTI within 1 year, and UTIs were associated with a total all-cause increased cost of $7045.00 per person per year [Yu S et al. J Diabetes Complications. 2014].
SGLT2i-induced glycosuria raises the risk of developing genital infections and, to a lesser extent, UTIs [Geerlings S et al. Diabetes Res Clin Pract. 2014]. In existing data, however, a definitive dose relationship between the incidence of these infections and the doses of SGLT2 is not evident. Most UTIs are mild and easily treated, whereas treatment of ASB is not warranted. Balanitis in men is relevant only in those who are prone to recurrent balanitis, in which case topical prophylaxis before SGLT2i initiation may be warranted. Most cases of candida vaginitis are mild and respond to therapy; the condition is relevant only in women with persistent symptoms or infection, or with recurrent symptoms. In such cases, consider avoiding all SGLT2i or using suppressive fluconazole therapy, although candida glabrata will not respond to this therapy.
Prof Sobel concluded that a modest increase in UTIs in patients with T2DM treated with SGLT2i therapy can be expected, can be easily managed, and is not a contraindication to therapy. Mild-to-moderate mycotic genital infections in both sexes are more frequent with SGLT2i therapy and are generally easily managed with standard therapy.
- © 2014 MD Conference Express®