Summary
Empagliflozin is superior to glimepiride as an add-on treatment option for patients with type 2 diabetes mellitus who have not achieved good glycemic control on metformin, according to results from the Efficacy and Safety of Empagliflozin (BI 10773) With Metformin in Patients With Type 2 Diabetes trial that are presented in this article.
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
Empagliflozin is superior to glimepiride as an add-on treatment option for patients with type 2 diabetes mellitus (T2DM) who have not achieved good glycemic control on metformin, according to results from the Efficacy and Safety of Empagliflozin (BI 10773) With Metformin in Patients With Type 2 Diabetes trial [Ridderstråle M et al. Lancet Diabetes Endocrinol. 2014], announced lead investigator Martin Ridderstråle, MD, Steno Diabetes Center, Gentofte, Denmark.
Metformin represents traditional first-line therapy for patients with T2DM, but there is no consensus regarding optimal second-line treatment. According to Prof Ridderstråle, empagliflozin is a selective sodium-glucose cotransporter 2 inhibitor, a new class of drugs for the management of T2DM, while glimepiride is a sulfonylurea.
This phase 3 double-blind study aimed to compare the efficacy and safety of empagliflozin and glimepiride as second-line therapy in patients with T2DM who are not adequately controlled on metformin. The study included patients > 18 years with a body mass index ≤ 45 kg/m2 and with HbA1c levels between 7% and 10% who were on a stable background therapy of metformin for ≥ 12 weeks before randomization. The investigators excluded subjects with an estimated glomerular filtration rate < 60 mL/min/1.73 m2 during screening or placebo run-in.
The researchers randomized participants (n = 1549) in a 1:1 ratio to oral empagliflozin (25 mg, QD) or oral glimepiride (1 to 4 mg, QD) as add-on to metformin for 104 weeks. The primary end point was the change from baseline in HbA1c levels at weeks 52 and 104 and included noninferiority and superiority criteria.
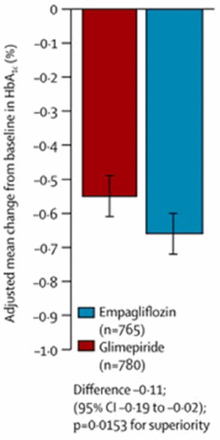
From baseline to 104 weeks, patients treated with empagliflozin had significantly greater reductions in mean HbA1c levels when compared with patients treated with glimepiride. Empagliflozin was noninferior to glimepiride at baseline and 104 weeks. The adjusted mean difference in change from baseline in HbA1c with empagliflozin versus glimepiride was −0.11% (95% CI, −0.19 to −0.02; P = .015 for superiority; see Figure 1). The investigators also observed significant reductions with empagliflozin in body weight (−3.1 vs 1.3 kg; P < .001), systolic blood pressure (−3.1 vs 2.5 mm Hg; P < .001), and diastolic blood pressure (−1.8 vs 0.9 mm Hg; P < .001). The incidence of death was similar in the 2 groups (0.7% vs 0.6%).
Change in Mean HbA1c From Baseline at 104 Weeks
Reprinted from The Lancet Diabetes & Endocrinology, 2, Ridderstrale M et al, Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial, 691–700, Copyright 2014, with permission from Elsevier.
Empagliflozin was also associated with significantly fewer hypoglycemic adverse events (AEs) than glimepiride (24% vs 3%; P < .001). In the empagliflozin group, serious AEs were reported in 16% of patients, compared with 11% in the glimepiride group. The incidence of AEs leading to treatment discontinuation was similar in both groups (5% vs 4%). Urinary tract infection was recorded in 13.7% and 13.1% of patients receiving empagliflozin and glimepiride, respectively, and genital infection in 11.8% and 2.2% of patients, respectively.
Prof Ridderstråle concluded that, compared with glimepiride, empagliflozin as add-on therapy to metformin produced a small but significantly superior difference in the reduction of HbA1c and provided sustained reductions in body weight and blood pressure. Patients treated with empagliflozin had fewer AEs, particularly hypoglycemia.
- © 2014 MD Conference Express®