Summary
Until recently, the only ways to reverse the action of nondepolarizing muscle relaxant were with neostigmine or other anticholinesterase drugs. Now, a new option—sugammadex—has come on the market in Europe, Japan, and China. This article discusses the safety and efficacy of neostigmine.
- Neurological Autoimmune Diseases
- Neuromuscular Blockade
- Analgesic Drugs
- Anesthesiology
- Neurological Autoimmune Diseases
- Neuromuscular Blockade
- Analgesic Drugs
Until recently, the only ways to reverse the action of nondepolarizing muscle relaxant were with neostigmine or other anticholinesterase drugs. Now, a new option—sugammadex—has come on the market in Europe, Japan, and China. Glenn S. Murphy, MD, University of Chicago Pritzker School of Medicine, Chicago, Illinois, USA, spoke on the safety and efficacy of neostigmine. Thomas Fuchs–Burder, MD, University of Lorraine, Nancy, Lorraine, France, reviewed the use of sugammadex in Europe.
Neostigmine has numerous side effects, such as bradycardia, nausea, and vomiting [Schaller SJ, Fink H. Core Evid. 2013]. According to Dr Murphy, other side effects include an inability to reverse deep levels of blockade, a ceiling effect, slow onset, and paradoxical muscle weakness. It also frequently fails to fully reverse muscular blockade.
In a prospective cohort study on residual paralysis, 134 of 150 patients who were given neuromuscular blocking drugs received neostigmine [Thilen SR et al. Anesthesiology. 2012]. Patients with intraoperative train–of–four (TOF) monitoring of eye muscles had a significantly greater incidence of residual paralysis than patients monitored at the adductor pollicis (P < .01). Residual paralysis was seen in 52% and 22% of patients, respectively. In both cases, TOF was < 0.9.
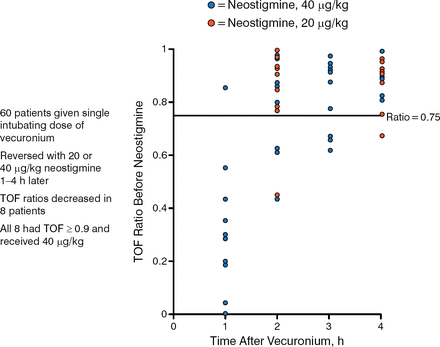
Another study evaluated the efficacy of 20 vs 40 μg/kg of neostigmine in 60 patients who received a single intubating dose of vecuronium [Caldwell JE. Anesth Analg. 1995]. One to 4 hours later, TOF ratios decreased in 8 patients. All had a TOF ratio ≥ 0.9 and received 40 μg/kg. The incidence of cardiovascular effects was high (50%) after both doses (Figure 1).
Reversal of Residual Neuromuscular Block With Neostigmine
TOF, train–of–four.
Reproduced from Caldwell JE. Reversal of residual neuromuscular block with neostigmine at one to four hours after a single intubating dose of vecuronium. Anesth Analg. 1995;80:1168–1174. With permission from International Anesthesia Research Society.
The introduction of sugammadex into clinical practice has revolutionized the way that anesthesiologists think about drug reversal [Schaller SJ, Fink H. Core Evid. 2013]. As the first cyclodextrin to be used as a therapeutic agent, it quickly, effectively, and safely reverses steroidal neuromuscular blockers by encapsulating the muscle relaxant and rendering it inactive [Jahr JS et al. Am J Ther. 2014].
Since its approval in Europe in 2008, thousands of doses have been given. The drug has been used extensively across a range of special populations—including pregnant women, elderly individuals, and patients with myasthenia gravis [Schaller SJ, Fink H. Core Evidence. 2013]—with no unexpected adverse reactions [Gold SJA, Harper NJN. Trends in Anesthesia and Critical Care. 2012].
Among those with morbid obesity, sugammadex enabled a safer and faster recovery from profound rocuronium–induced neuromuscular blocking than neostigmine (Table 1) [Carron M et al. Obes Surg. 2013]. Another investigation found that pretreatment with a single intravenous dose of magnesium (60 mg/kg) did not decrease the efficacy of sugammadex for the reversal of a moderate and deep neuromuscular block induced by an intubation dose of rocuronium [Czarnetzki C et al. Anesthesiology. 2014].
Anesthetics, Anesthesiology, and Surgical Times by Group
Sugammadex is a fast, potent, and safe reversal agent for aminosteroid muscle relaxants. Although it has yet to be approved by the US Food and Drug Administration, it clearly has the potential to be a worldwide game changer in the practice of anesthesia.
- © 2014 MD Conference Express®