Summary
Treatment of articular cartilage and joint damage as a result of acute injury is challenging. This article addresses the particular topics of the basic science related to the early response of cartilage after traumatic injury, current and future chondroprotective products, osteochondral allografts, as well as chondroplasty, microfracture, and cells in relation to articular cartilage injuries.
- Orthopaedic Procedures
- Sports MedicineTrauma
- Orthopaedic Procedures
- Sports Medicine
- Orthopaedics
- Trauma
Treatment of articular cartilage and joint damage as a result of acute injury is challenging. Dominik Haudenschild, PhD, University of California Davis Medical Center, Sacramento, California, USA, discussed the basic science related to the early response of cartilage after traumatic injury.
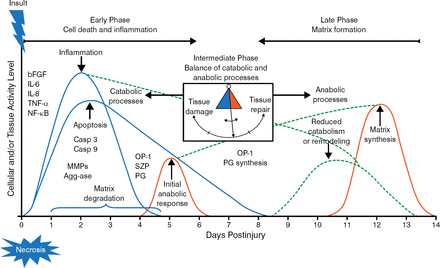
Although there is no cure for osteoarthritis (OA), and most OA is idiopathic, about 50% of patients who have a history of meniscus or anterior cruciate ligament injuries will develop OA in 5 to 20 years [Lohmander LS et al. Am J Sports Med. 2007]. The current knowledge of acute trauma includes an early phase consisting of cell death and inflammation and a late phase that consists of matrix formation (Figure 1) [Anderson DD et al. J Orthop Res. 2011]. The early phase is a catabolic stage that involves gene expression changes that drive inflammation, apoptosis, and matrix degradation, whereas the late phase is marked by reduced catabolism and upregulation of anabolic processes to synthesize a new matrix. The focus of Dr Haudenschild's research is to identify and reduce the early catabolic responses to joint injury, with the hope that this will protect the joint against OA in the long term.
Cellular Responses to Acute Knee Trauma
bFGF, basic fibroblast growth factor; Casp, caspase; IL, interleukin; MMP, matrix metalloproteinase; NF-κB, nuclear factor-κB; OP-1, osteogenic protein-1; PG, proteoglycan; SZP, superficial zone protein; TNF-α, tumor necrosis factor-α.
Adapted from Anderson DD et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. With permission from the Orthopaedic Research Society.
In a mouse model, noninvasive knee injury results in multiple events that mimic a human knee injury, including cartilage tears, changes in the meniscus and subchondral bone, synovial hyperplasia, loss of proteoglycans, loss of surface lamina, and consistent OA within 8 weeks [Christiansen BA et al. Osteoarthritis Cartilage. 2012]. In the mouse model, up to 100-fold changes in gene expression occur within 1 to 2 hours of the injury, with changes in small metabolites occurring by 1 hour. Bone remodeling is detectable within 3 days. The goal of ongoing research is to develop therapeutic strategies that reduce the cellular contributions to joint degradation and OA progression, by identifying and characterizing the early responses to injury.
Susan Chubinskaya, PhD, Rush University Medical Center, Chicago, Illinois, USA, discussed current and future chondroprotective products. Articular cartilage injury is prevalent in about 48% of athletes, with 86% experiencing symptoms. Importantly, articular cartilage does not heal, and it is hard to duplicate because it is thin, resists compression, is able to distribute a load, and has a low coefficient of friction [Flanigan DC et al. Med Sci Sports Exerc. 2010].
The current treatment options for cartilage injury include surgical repair (eg, debridement, marrow stimulation, and osteochondral transfer) and cell-based cartilage repair (eg, chondrocyte transplantation or implantation) [Mithoefer K et al. Clin Sports Med. 2009]. Other options include biologic interventions in the setting of a clinical trial and cartilage-tissue engineering. Experimental approaches to cartilage repair include applying pulsed electromagnetic fields or ultrasound therapy, implanting acellular or cellular scaffolds, injecting hydrogels or nano-particles with active agents, and transplanting periosteum.
The goal of biologic therapy is to promote cartilage repair through anti-inflammatory, chondroprotective, matrix-protective, or anabolic methods. Chondrocytes and stem cells secrete multiple growth factors that promote these processes. In a randomized, double-blind, placebo-controlled trial of patients with primary knee OA scheduled for total knee replacement, treatment with the growth factor recombinant human fibroblastic growth factor 18 (rhFGF-18) resulted in improvement in cartilage histology, OA severity, and cartilage biomechanical properties [Dahlberg LE et al. Osteoarthritis Cartilage. 2011]. Joint-space narrowing and cartilage thickness remained similar among the rhFGF-18 and placebo arms, and there were no safety concerns or systemic effects. Treatment of an animal model with the growth factor bone morphogenetic protein-7/osteogenic protein-1 resulted in improvement in osteochondral and chondral defects, and mosaicplasty [Hayashi M et al. Arthritis Res Ther. 2008]. Multiple clinical trials are ongoing to evaluate the role of biologic therapy in articular cartilage defects.
Seth I. Gasser, MD, Florida Orthopaedic Institute, Tampa, Florida, USA, discussed chondroplasty, microfracture, and cells in relation to articular cartilage injuries. When treating articular cartilage injuries, it is important to consider patient-specific factors such as age and activity level, as well as lesion-specific factors such as etiology, grade, and location.
Arthroscopic debridement/chondroplasty is an inexpensive procedure that is suited for low-grade lesions; however, it does not stimulate healing, and normal cartilage may be removed [McCormick F et al. Arthroscopy. 2014]. Microfracture is best for patients aged < 55 years who have small full-thickness defects, and it results in fibrocartilage resurfacing of the lesion; however, fibrocartilage does not have the same mechanical characteristics as hyaline cartilage, and subchondral cysts and osteophytes can develop [Minas T et al. Am J Sports Med. 2009]. In a long-term study of 72 patients aged < 45 years (range, 13 to 45 years) with traumatic chondral defects, 80% reported improvement at year 7 postoperation, with improvement in function and pain [Steadman JR et al. Arthroscopy. 2003]. At final follow-up, 32% indicated that they were pain free.
Mosaicplasty is indicated in grade III to IV lesions; however, it is limited by imprecise contouring, fibrocartilage development between donor hyaline cartilage plugs, and structural differences. In a study of > 1000 mosaicplasties, good-to-excellent results were reported at 5 years in lesions located at the femoral condyle (92%), tibia (87%), patella/trochlea (74%), and talar (93%) [Hangody L et al. Injury. 2008]. However, another study found no significant difference between osteoarticular transfer system and autologous chondrocyte implantation (ACI) procedures [Dozin B et al. Clin J Sports Med. 2005]. ACI is limited by its high cost, uneven distribution of cells, and chondrocyte dedifferentiation. Other potential complications include arthrofibrosis and graft failure [Niemeyer P et al. Am J Sports Med. 2008]. In addition, 2 studies have demonstrated that ACI produces similar outcomes as microfracture at 1- and 5-year follow-up [Saris DB et al. Am J Sports Med. 2008; Knutsen G et al. J Bone Joint Surg Am. 2007].
Emerging treatment options include bone marrow aspirate concentrate to deliver mesenchymal stem cells, autologous matrix-induced chondrogenesis to provide a scaffold to microfracture, and juvenile particulated articular cartilage.
James P. Stannard, MD, University of Missouri, Columbia, Missouri, USA, presented more detail about osteochondral allografts. The limitations of allografts are availability and survival of the graft, as well as difficulties during implantation. One problem with allografts is the typical storage conditions, which can compromise the graft. Current conditions store the graft at 4°C, and mandatory disease screening prevents use of the graft before 14 days. The Mizzou Tissue Preservation System stores the grafts at 25°C. After 30 and 60 days of storage, the viability of grafts treated by the Mizzou system was 91% and 86%, respectively, compared with 78% and 26% for grafts stored according to the standard of care [Cook JL et al. Clin Orthop Relat Res. 2014]. In a human validation study of the Mizzou system, grafts treated according to the Mizzou system were viable up to day 70, whereas grafts treated according to the standard of care contained mostly dead cells by day 56 [Stoker AM et al. Orthop Res Soc. 2015].
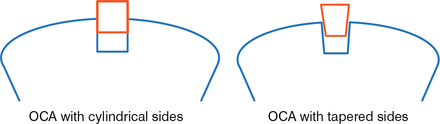
Another limitation of osteochondral allografts is challenges associated with their implantation. Graft chondrocytes can be damaged due to the high force required to implant cylindrical grafts [Kang RW et al. Am J Sports Med. 2010]. However, tapering the sides of the osteochondral allograft reduces the frictional resistance during implantation of the graft, which may reduce the damage to graft chondrocytes (Figure 2). In a study comparing graft shape, total cell viability was not affected by graft shape; however, superficial cell death was significantly greater in cylindrical plugs compared with tapered plugs (P = .003). In addition, the force required to insert the tapered plug was significantly less than that used for cylindrical plugs (P = .012) [Pfeiffer FM et al. Orthopaedic Research Society. 2013].
Shape of Osteochondral Allografts Affects Chondrocyte Damage
OCA, osteochondral allograft.
Reproduced with permission from JP Stannard, MD.
Christian Krettek, Hannover Medical School, Hannover, Germany, discussed joint preservation treatment of intra-articular malunions. In these cases, there are a number of options to consider for treatment, including a “wait and see” approach, cartilage repair, osteotomies, arthroplasty, and amputation. There are little data reported in the literature regarding treatment of intra-articular and intra-condylar malunion. Due to a lack of knowledge regarding treatment of these deformities, it is important to understand the symptoms and pathology that the patient is experiencing, which are often a combination of issues including alignment, stability, and congruency. It is also important to analyze the malunion and thoroughly plan an approach or method to treat it.
In conclusion, although there are challenges associated with the treatment of articular cartilage and joint trauma, there are current and emerging treatment options that may be beneficial for patients. It is important to consider patient- and lesion-specific characteristics, as well as the planned approach for optimal outcomes to be achieved.
The editors would like to thank the many members of the 2014 Orthopaedic Trauma Association Annual Meeting presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®