Summary
Recommended diagnostic protocols of bronchiectasis assume that patients are alike, which can be questioned, especially when many tests are recommended for diagnosis. Evaluation is influenced by factors including age, disease severity and character, and the resources available to perform the diagnostic tests. This article discusses the systematic evaluation of these patients and treatment strategies for non-CF bronchiectasis.
- Lower Respiratory Infections
- Pulmonary & Respiratory Medicine
- Lower Respiratory Infections
Mark Metersky, MD, University of Connecticut School of Medicine, Farmington, Connecticut, USA, discussed the systematic evaluation of patients with bronchiectasis. Recommended diagnostic protocols assume that patients are alike, which can be questioned, especially when many tests are recommended for diagnosis. Evaluation is influenced by factors including age, disease severity and character, and the resources available to perform the diagnostic tests.
The list of reported etiologies of bronchiectasis is long, and the cause is unknown in about 50% of patients. Infections are a relatively frequently identified cause [Quast TM et al. Dis Mon. 2008] and include allergic bronchopulmonary aspergillosis and Mycobacterium avium complex. Idiopathic causes may include an unrecalled previous infection, or deficiencies in heterozygote α-1 antitrypsin or immunoglobulin G subclass; other causes are listed in Table 1.
Causes of Idiopathic Bronchiectasis
Determining the etiology, however challenging, can help patients understand their condition, which can be valuable in genetic counseling. A definitive diagnosis can also guide therapy [Pasteur MC et al. Am J Respir Crit Care Med. 2000; Shoemark A et al. Respir Med. 2007]. Imaging can be useful in diagnosis.
Patient characteristics of note include age, with younger patients being more likely to have a congenital cause; previous severe infection; autoimmune disease; paranasal sinus diseases; and fertility issues.
Recommended tests for all patients include high-resolution computed tomography; pulmonary function; examination of sputum for bacteria, acid-fast bacilli, and fungus; blood count; and quantification of immunoglobulin classes [Metersky ML. Clin Chest Med. 2012]. In the absence of an etiology, determination of α-1 antitrypsin and screening for CF are prudent courses. The relationship of α-1 antitrypsin deficiency with bronchiectasis is contentious. Whereas some studies have not reported increased prevalence of α-1 antitrypsin deficiency in patients with bronchiectasis, a study of 74 patients with the deficiency reported bronchiectasis in 70 [Parr DG et al. Am J Respir Crit Care Med. 2007]. Newly identified patients with bronchiectasis should be evaluated using a selection of tests.
CURRENT TREATMENT OPTIONS FOR NON-CYSTIC FIBROSIS BRONCHIECTASIS
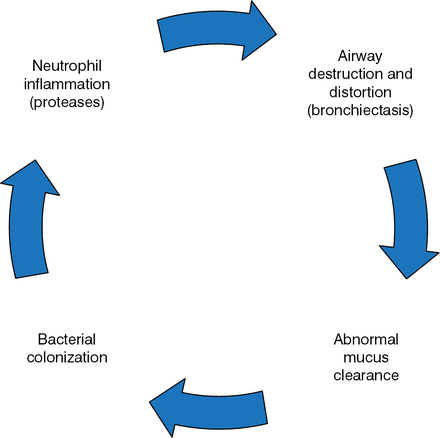
Ashwin Basavaraj, MD, New York University Langone Medical Center, New York, New York, USA, discussed treatment strategies for non-CF bronchiectasis, starting with the vicious cycle hypothesis (Figure 1).
The Vicious Cycle of Bronchiectasis
Reprinted with permission of the American Thoracic Society. Copyright © 2014 American Thoracic Society. McShane PJ et al. Non-Cystic Fibrosis Bronchiectasis. Am J Respir Crit Care Med. 2013;188:647–656. Official Journal of the American Thoracic Society.
Airway clearance options include physiotherapy (high-frequency chest wall oscillation, positive expiratory pressure, posture-related drainage, and manual manipulation of the chest) and use of inhaled hypertonic saline or mannitol. These approaches appear safe in those with stable bronchiectasis, although their roles in cases of acute exacerbations are unclear, and the relative merits of each airway clearance option need to be explored [Lee AL et al. Cochrane Database Syst Rev. 2013].
The use of inhaled mannitol to hydrate the mucus and promote its clearance has been explored in several randomized trials. One reported reduced plugging of small airways by mucus in mannitol-treated patients, although there were no differences in quality of life (QOL), exercise capacity, spirometry, inflammatory markers, or bacterial load between the treated and placebo arms [Bilton D et al. Chest. 2013]. In another study, no difference in annual rate of exacerbations was evident in patients who inhaled 400 mg (n = 233) or 50 mg (n = 228) of mannitol twice daily for 52 weeks, although the higher dose was associated with significant improvements in time to first exacerbation and QOL [Bilton D et al. Thorax. 2014].
Pulmonary rehabilitation can aid in airway clearance, providing short-term improvement in exercise capacity and QOL; whether the benefits persist is unclear [Lee AL et al. Respir Res. 2014]. Drug therapies of potential benefit include atorvastatin [Mandal P et al. Lancet Respir Med. 2014] and formoterol-budesonide [Martinez-Garcia MA et al. Chest. 2012].
The use of macrolide antibiotics suppresses inflammatory mediators, modifies the production of mucus, and may reduce biofilm formation. The EMBRACE trial [Wong C et al. Lancet. 2012] that evaluated the macrolide antibiotic azithromycin reported a benefit in terms of time to first exacerbation, decreased C-reactive protein at 6 months, and increased forced vital capacity at 6 and 12 months. The BLESS trial [Serisier DJ et al. JAMA. 2013] evaluated low-dose erythromycin twice daily for 12 months versus placebo. While the rate of pulmonary exacerbations was decreased, increased macrolide resistance tempered the significance of the findings.
Antibiotics are another option in bronchiectasis treatment. Inhalation is a potentially effective route of delivery [Brodt AM et al. Eur Respir J. 2014; Haworth CS et al. Am J Respir Crit Care Med. 2014; Serisier DJ et al. Thorax. 2013]. However, in 2 double-blind, randomized, placebo-controlled trials, the delivery of aztreonam via inhalation was not effective [Barker AF et al. Lancet Respir Med. 2014].
Surgery, such as lobectomy, pneumonectomy, and segmentectomy, is an option for patients who are nonre-sponsive or intolerant to the aforementioned therapies, and can help resolve complications including recurrent infection, empyema, or hemoptysis [Balci AE et al. Ann Thorac Surg. 2014; Mitchell JD et al. Ann Thorac Surg. 2012]. The results can be improvement of symptoms and QOL.
TREATMENTS ON THE HORIZON FOR NON-CF BRONCHIECTASIS
Anne O'Donnell, MD, Georgetown University, Washington, DC, USA, provided a look ahead at treatments for non-CF bronchiectasis. Treatment is governed by 4 treatment tenets: Educate patients, understand the disease's impact, understand the extent of the disease, and target organisms. Goals are to reduce exacerbations, control symptoms, improve QOL, preserve lung function, and reduce mortality.
Essentially, all therapies for non-CF bronchiectasis are new, because none have been approved by the Food and Drug Administration. Treatments target the underlying disease if possible, with the goals of clearing airways, controlling inflammation, and treating exacerbations. Avenues being explored in treating the underlying disease include using CF-targeted drugs like ivacaftor, replacement of α-1 antitrypsin or immunoglobulin, and addressing vitamin D deficiency. The latter has been linked to bronchiectasis [Chalmers JD et al. Thorax. 2013], but Dr O'Donnell noted that no clinical trials were underway.
Concerning anti-inflammatory therapies, the use of macrolides has yielded promising results, although development of resistance is a problem. Inhaled steroids, phosphodiesterase 4 inhibitors, and statins are all potentially beneficial. The neutrophil elastase inhibitor AZD9668 was well tolerated and showed promise in a small phase 2 study in terms of improved lung function and prognostic biomarkers [Stockley R et al. Respir Med. 2013].
Antibiotics delivered intravenously or via inhalation have shown potential in numerous studies, but further, larger studies need to be done. The use of inhaled aztreonam was not found to confer benefit [Barker A et al. Eur Respir J. 2013]. Antibiotic therapy targeting Pseudomonas [White L et al. Respir Med. 2012] and an intensive course of intravenous antibiotics [Mandal P et al. QJM. 2013] have also shown promise.
The take-home message of the future of non-CF bronchiectasis therapy is potential, with further studies necessary to proceed toward clinical use.
- © 2014 MD Conference Express®