Summary
President of the American Heart Association (AHA), Elliott Antman, MD, Harvard Medical School, Boston, Massachusetts, USA, gave the Presidential Address at the opening of the AHA 2014 Scientific Sessions. He focused on how to best utilize emerging technologies and use big data to improve patient outcomes. With new diagnostic and therapeutic options being discovered at an unprecedented pace, there is a likewise unprecedented opportunity to save and improve lives.
- prevention & screening
- cardiology genomics
President of the American Heart Association (AHA), Elliott Antman, MD, Harvard Medical School, Boston, Massachusetts, USA, gave the Presidential Address at the opening of the AHA 2014 Scientific Sessions. He focused on how to best utilize emerging technologies and use big data to improve patient outcomes. With new diagnostic and therapeutic options being discovered at an unprecedented pace, there is a likewise unprecedented opportunity to save and improve lives.
Heart disease and stroke remain the leading causes of death across the globe, resulting in > 17 million deaths per year [World Health Federation. Cardiovascular Diseases Fact Sheet. 2013]. This number is expected to rise to > 20 million by 2030. The World Heart Federation has a 2025 goal to reduce premature deaths from cardiovascular disease (CVD) by 25% [Smith SC et al. J Am Coll Cardiol. 2012], and the AHA 2020 Impact Goal seeks to increase cardiovascular (CV) health by 20% and reduce CV death by 20% [AHA. 2013 Statistical Fact Sheet: 2020 Impact Goal. 2013]. To meet these goals, new technologies are needed to accelerate discovery of new therapeutics.
Dr Antman believes that dramatic advances in treatments for CVD will require disruptive innovation. The concept of disruptive innovation was first introduced by Harvard Business School professor Clayton Christensen, and it refers to the appearance of a new technology that unexpectedly results in extraordinary changes in the way that people live and work. One example is the evolution from the landline telephone to the cellular phone to today's smartphones.
Dr Antman shared an anecdote about one of his patients who had been experiencing recurrent palpitations. The palpitations were not diagnosed despite multiple electrocardiograms, ambulatory monitoring, and exercise testing. Dr Antman prescribed a portable heart rhythm-monitoring device that latches on to a smartphone, which the patient was able to use to record and e-mail tracings of his heart rhythm. Upon review of the tracings, Dr Antman diagnosed the patient with atrial fibrillation and formulated a therapeutic plan.
The development of warfarin is another example of disruptive innovation. In 1933, a Wisconsin farmer noticed that after eating sweet clover hay, some of his dairy cows developed bleeding problems that were often fatal. He took the hay to agricultural biochemist Karl Paul Link at the University of Wisconsin. Link and his team were familiar with coumarin, which is present on the shaft of sweet clover hay. They discovered that when this type of hay became wet, a fermentation process fused 2 molecules of coumarin (dicoumarol), producing the anticoagulant that sickened the cows. Recognizing the significance of this, Link and his colleagues created the potent synthetic derivative warfarin.
In contrast to the serendipitous discovery of warfarin, the latest generation of novel oral anticoagulants (eg, dabigatran, rivaroxaban, apixaban, edoxaban) underwent targeted, long, and expensive development processes. Bringing a new therapy to market today takes approximately 15 years and costs about US$1 billion. To improve assessment of new treatments in clinical trials and the discovery/preclinical phase of new treatments, it would be beneficial to use a systems medicine approach in drug discovery where integrated genetic, molecular, and cellular data are used to build a model to predict the effect of a new therapy on an individual patient, ultimately resulting in a range of customized therapies.
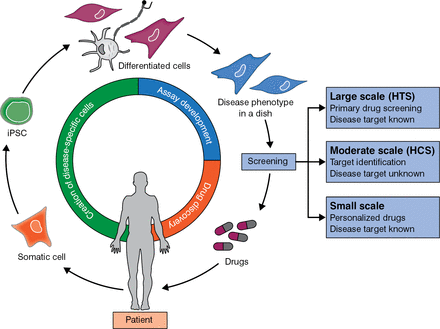
Two breakthroughs may help in the quest to provide patients with individualized therapy. Prof Shinya Yamanaka found that human skin fibroblasts can be reprogrammed into pluripotent stem cells [Takahashi K et al. Cell. 2007], which can be differentiated into specific cell types, such as cardiomyocytes. In a similar fashion, cells from patient biopsies can be used to screen drugs and ascertain the best therapy for a particular patient (Figure 1).
From Patient Biopsy Cells to Individualized Medicine
HCS, high-content screening; HTS, high-throughput screening; iPSC, induced pluripotent stem cell.
Adapted from Mercola M et al. Induced pluripotent stem cells in cardiovascular drug discovery. Circ Res. 2013;112:534–548. With permission from the American Heart Association.
A “heart on a chip” is another recent in vitro model that may advance the development of personalized medicine [McCain ML et al. PNAS. 2013]. In this bioengineering platform, neonatal rat ventricular myocytes are layered on a deformable, thin, elastic chip. Upon contraction of the myocytes, the chip bends. Drugs can be tested by placing the chip in a microfluidic test chamber and providing an electrical current to stimulate the cells.
Antman then looked toward new technologies that also hold promise for clinical research. The availability of wearable sensors that provide data to an Internet database via a smartphone has presented researchers with new ways to evaluate therapies and monitor outcomes. The University of California at San Francisco Health eHeart Study (www.health-eheartstudy.org), an online research database, links study participants' wireless sensors and will eventually link their data to electronic medical records. Information such as activity level, habits, medical history, and outcomes will be collected. The AHA is collaborating with the Health eHeart Study and encouraging participants from AHA programs to enroll. Appropriate data security measures have been put into place to protect participant privacy.
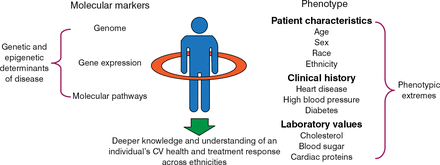
The Cardiovascular Genome-Phenome Study (CVGPS) is another example cited by Antman of using new technologies in clinical research [Benjamin I et al. Circulation. 2014]. The CVGPS is a collaboration of the AHA and the academic institutions of the Framingham Heart Study and the Jackson Heart Study. This revolutionary study will change clinical research by combining findings from the Framingham, Jackson, and other important cohort studies with genomic and phenomic data. As illustrated in Figure 2, results from the CVGPS will, ideally, provide additional understanding of the biology underlying phenotypic extremes, including patients affected early in life, those who are severely affected, and those who remain healthy. Genetic and epigenetic determinants, epidemiologic differences, and treatment response across ethnicities will be included.
CVGPS: 360° Look at Cardiovascular Health
CV, cardiovascular; CVGPS, Cardiovascular Genome-Phenome Study.
Reproduced with permission from E Antman, MD.
CVGPS investigators will also provide a biorepository and determine the best electronic health approaches to digital data collection. The first 8 investigators of the CVGPS were introduced during the presentation (Ramy Arnaout, Donna Arnett, Christy Avery, Susan Cheng, John Cole, Simin Liu, George O'Connor, and Marc Vidal). Dr Antman stated, “They are building the future on the power of the past and are following in the footsteps of the AHA founders in a bold and novel way.”
The editors would like to thank the many members of the American Heart Association Scientific Sessions 2014 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®