Summary
Although tumor necrosis factor inhibitors (TNFis) have been shown to be effective in the treatment of rheumatoid arthritis, little is known about the effect of stopping this therapy in patients with stable low disease activity, particularly with respect to the likelihood of disease flare, and whether TNFis can be restarted effectively and safely. This article looks at the multicenter, open-label, randomized, Potential Optimalization of (Expediency) and Effectiveness of TNF-blockers trial [POET; NTR3112].
- Rheumatoid Arthritis
- Rheumatology Clinical Trials
- Rheumatoid Arthritis
- Rheumatology Clinical Trials
- Rheumatology
Harald E. Vonkeman, MD, PhD, Medisch Spectrum Twente, Enschede, The Netherlands, shared data from the multicenter, open-label, randomized, Potential Optimalization of (Expediency) and Effectiveness of TNF-blockers trial [POET; NTR3112]. The results showed that, in patients with rheumatoid arthritis (RA) who have stable low disease activity, tumor necrosis factor inhibitors (TNFis) can be abruptly withdrawn without the disease flaring. If a disease flare does occur, TNFi therapy can once again be effectively restarted.
According to Prof Vonkeman, although TNFis have been shown to be effective in the treatment of RA, little is known about the effect of stopping this therapy in patients with stable low disease activity, particularly with respect to the likelihood of disease flare, and whether TNFis can be restarted effectively and safely. He added that, due to the risk of serious side effects and complications in patients who take TNFis and their high cost, it is important to know whether individuals in stable low disease activity can effectively stop their TNFi therapy.
The POET study therefore aimed to determine whether patients with RA in stable low disease activity can stop their TNFi therapy. It included 817 patients from 47 centers, all of whom had low RA disease activity and had been treated with a TNFi for ≥ 12 months. During the 6 months prior to the study, all patients had also received a stable dose of disease-modifying antirheumatic drug(s) and had ≥ 2 Disease Activity Score in 28 joints (DAS28) scores < 3.2 in this period. Patients were randomized 2:1 to either stop (65%) or continue (35%) their TNFi therapy (DAS28 flare; defined as DAS28 ≥ 3.2 with an increase ≥ 0.6 compared with the previous DAS28).
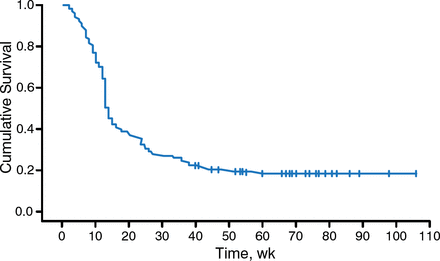
At 12 months, the data showed that 46.9% of patients who stopped their TNFi experienced a DAS28 flare, compared with 16.6% of those who continued their TNFi (P < .001). The median time to first flare was 24 weeks in patients who stopped taking their TNFi. However, most patients in the group who stopped their TNFi regained low disease activity quickly after a flare, at a median time of 14 weeks (Figure 1).
Time to Regained Low Disease Activity After Flare in Patients Who Stopped TNFi Therapy
TNFi, tumor necrosis factor inhibitor.
Reproduced with permission from HE Vonkeman, MD, PhD.
During the 12-month study period, flare-free discontinuation of TNFi was possible in 53% of patients with stable low RA disease activity.
Overall, these data demonstrate that abrupt discontinuation of TNFi can be safely and effectively implemented in this patient population. Additionally, if a disease flare is going to occur after TNFi withdrawal, it will occur soon, and patients can effectively restart their therapy with restoration of low disease activity, on average, by 14 weeks.
- © 2014 MD Conference Express®