Summary
This article discusses the main results of the randomized BASKET Prospective Validation Examination II trial [BASKET-PROVE II], which compared the long-term outcome of biodegradable-polymer drug-eluting stents to both durable-polymer drug-eluting stents and bare metal stents.
- BASKET-PROVE
- cardiology clinical trials
- interventional techniques & devices
Christoph A. Kaiser, MD, University Hospital Basel, Basel, Switzerland, discussed the main results of the randomized BASKET Prospective Validation Examination II trial [BASKET-PROVE II; Kaiser C et al. Circulation. 2014], which compared the long-term outcome of biodegradable-polymer drug-eluting stents (BP-DEs) to both durable-polymer drug-eluting stents (DP-DESs) and bare metal stents (BMSs).
In BASKET-PROVE II, 2291 patients requiring ≥ 3.0-mm stents were randomized between April 2010 to May 2012 in a 1:1:1 fashion to either a biolimus-eluting BP-DES (Nobori; n = 765), an everolimus-eluting DP-DES (Xience-PRIME; n =765), or a thin-strut coated cobalt-chromium BMS (Prokinetik; n = 761). Patients with shock, in-stent restenosis, stent thrombosis (ST), unprotected left main or saphenous vein graft, planned surgery within 12 months, increased bleeding risk due to oral anticoagulant, and history of stroke or transient ischemic attack and who required stents > 4 mm in diameter were excluded.
The noninferiority margin for the BP-DES versus DP-DES comparison was 3.8%, based on prior findings [Kaiser C et al. N Engl J Med. 2010]. The primary efficacy end point during the 2-year follow-up after stent implantation was the occurrence of major adverse cardiac events (defined as cardiovascular [CV] death, myocardial infarction [MI], or target vessel revascularization). A superiority analysis was planned between the BP-DES and BMS using a secondary safety end point of CV death, MI, or definite/probable ST.
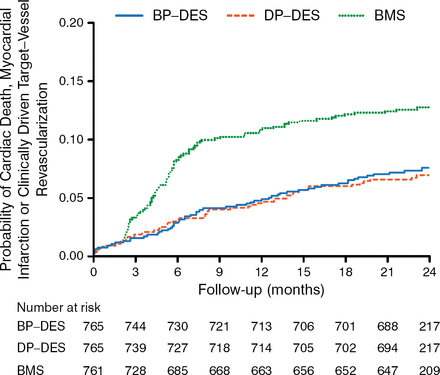
Baseline characteristics were comparable in the 3 trial arms. At 2-year follow-up, 98.5% of patients were alive and 97.7% of patients remained in follow-up. The primary end point was comparable in patients receiving BP-DES and those receiving DP-DES (2-year rate, 7.6% vs 6.8%; absolute risk difference, 0.75%; 95% CI, −1.93% to 3.50%; P Noninferiority = .04; HR, 1.11; 95% CI, 0.77 to 1.62; P = .58; Figure 1).
Primary End Point Between Drug-Eluting Stents
BMS, bare metal stent; BP-DES, biodegradable-polymer drug-eluting stent; DP-DES, durable-polymer drug-eluting stent.
Adapted from Kaiser C et al. Long-Term Efficacy and Safety of Biodegradable-Polymer Biolimus-Eluting Stents Main Results of the Basel Stent Kosten-Effektivitäts Trial-PROspective Validation Examination II (BASKET-PROVE II), A Randomized, Controlled Noninferiority 2-Year Outcome Trial. Circulation. E-pub ahead of print. DOI: 10.1161/CIRCULATIONAHA.114.013520. Accessed December 10, 2014. With permission from American Heart Association, Inc.
The results were consistent in the per-protocol population although it did not meet the prespecified noninferiority margin (absolute risk difference, 1.41%; 95% CI, 1.33% to 4.15%; P Noninferiority = .09). Both DES platforms had lower occurrence of target vessel revascularization as compared with BMS.
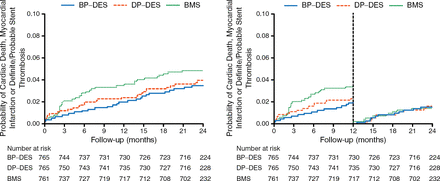
The secondary safety end point was similar for BP-DES as compared with BMS (3.7% vs 5.0%; HR, 0.72; 95% CI, 0.44 to 1.18; P = .20; left panel of Figure 2). A landmark analysis at 1 year revealed no difference in late safety between the 2 stents (right panel of Figure 2).
Key Safety Secondary End Point Between BP-DES and BMS
BMS, bare metal stent; BP-DES, biodegradable-polymer drug-eluting stent; DP-DES, durable-polymer drug-eluting stent.
Adapted from Kaiser C et al. Long-Term Efficacy and Safety of Biodegradable-Polymer Biolimus-Eluting Stents Main Results of the Basel Stent Kosten-Effektivitäts Trial- PROspective Validation Examination II (BASKET-PROVE II), A Randomized, Controlled Noninferiority 2-Year Outcome Trial. Circulation. E-pub ahead of print. DOI: 10.1161/CIRCULATIONAHA.114.013520. Accessed December 10, 2014. With permission from American Heart Association, Inc.
In summary, BP-DES were noninferior to DP-DES after 2 years in patients requiring large-vessel stents. Both were superior to BMS in efficacy. There was no evidence of superior safety of BP-DES beyond 1 year. The findings do not support the idea that polymers are key in the perceived association of DP-DES with very late ST, although the trial was not powered to definitively assess this.
- © 2014 MD Conference Express®