Summary
With the advent of novel therapies, the mean survival rate of patients with multiple myeloma is now about 10 years. Prior to the approval of the novel therapies, stem cell transplantation was considered the first-line therapy for younger patients with multiple myeloma. Current prospective trials should shed light on delaying stem cell transplantation in favor of first-line treatment, so as to avoid both acute and long term toxicities in the years to come.
- long-term management
- relapse
- stem cell transplantation
- timing
- Big 5 agents
The mean survival of patients with multiple myeloma (MM) has now approached 10 years with the advent of novel therapies [Mikhael JR et al. Mayo Clin Proc. 2013]. As a result, a long-term approach to management is required that addresses clone elimination and control of disease, because many patients will receive multiple lines of therapy [Rajkumar SV et al. Blood. 2011]. Joseph Mikhael, MD, MEd, Mayo Clinic Arizona, Scottsdale, Arizona, USA, presented a practical approach to the management of relapsed MM.
The Mayo Stratification for Myeloma and Risk-Adapted Therapy (mSMART) is a risk stratification approach that estimates survival time for patients with newly diagnosed, active MM [Mikhael JR et al. Mayo Clin Proc. 2013]. About 20% of patients are high risk, which is characterized by disease with a 17p deletion and translocation between chromosomes 14 and 16 and 14 and 20, as well as a high-risk signature according to gene expression profiling. About 20% of patients are intermediate risk, which is characterized by disease with a translocation between chromosomes 4 and 14, cytogenetic deletion at chromosome 13 or hypodiploidy, and plasma cell labeling index ≥ 3%. Most patients are standard risk (60%), which is disease characterized by hyperdiploidy and translocations between chromosomes 11 and 14, and 6 and 14. The estimated survival for these risk groups are 3, 4 to 5, and 8 to 10 years, respectively. A study that used the mSMART approach to guide therapy for transplant-eligible and -ineligible patients with MM found that patients frequently have multiple clones with variable drug sensitivity and that reemergence of drug-sensitive clones can occur. As a result, combination chemotherapy should be used and continuous suppression therapy should be considered. In addition, high-risk individuals have clones with unstable DNA, which raises the question that perhaps DNA-damaging agents should be avoided in this risk group.
When determining the course of therapy for relapsed MM, clinicians should consider 5 questions:
-
Does the patient need to be treated now?
-
Should the patient be retreated with a previous therapy?
-
Have the “Big 5” been used in the patient?
-
Have “add-on” agents been used for therapy?
-
Has an individualized, risk-stratified approach been used for the patient?
Patients with monoclonal gammopathy of undetermined significance or with asymptomatic or smoldering MM, and some patients with true MM, do not require immediate therapy [Rajkumar SV et al. Lancet Oncol. 2014]. To determine which patients should be treated, the SLiM CRAB criteria (60% plasmacytosis, light chains I/U > 100, 1 or more focal lesion on magnetic resonance imaging, calcium elevation, renal insufficiency, anemia, and bone disease) should be employed. For relapsed MM, most patients with slow indolent relapse can be managed with watchful waiting, whereas patients with rapid and aggressive relapse should receive immediate treatment with a combination therapy. Retreatment with a previous therapy is an option in some patients and should depend on the depth and duration of the first response.
When treating patients with relapsed MM, Dr Mikhael highlighted the Big 5, which are thalidomide, bortezomib, lenalidomide, carfilzomib, and pomalidomide. He pointed out that thalidomide is often overlooked in the United States but is a highly active agent in MM. Bortezomib is popular for the treatment of upfront and relapsed MM, and lenalidomide is commonly used for the treatment of relapsed disease. Carfilzomib was approved by the US Food and Drug Administration (FDA) in 2012, and a phase 2 trial demonstrated that carfilzomib monotherapy resulted in a partial response and stable disease in about 18% and 32% of patients, respectively, who had received ≥ 2 prior therapies and were refractive to the most recent regimen [Siegel DS et al. Blood. 2012]. The Phase 3 ASPIRE study demonstrated that carfilzomib plus lenalidomide and dexamethasone significantly prolonged progression-free survival (PFS) compared with lenalidomide plus dexamethasone (P < .0001) [Stewart AK et al. N Engl J Med. 2014]. However, carfilzomib monotherapy failed to meet the primary end point in the Phase 3 FOCUS study [Ludwig H et al. ESMO 2014 (abstr LBA28)]. Pomalidomide was approved by the FDA in 2013. In the MM-003 trial, pomalidomide plus low-dose dexamethasone significantly improved overall survival (OS) compared with high-dose dexamethasone in patients with refractory MM who did not respond to bortezomib and lenalidomide (HR, 0.53; P < .001) [Dimopoulos MA et al. Blood. 2012 (abstract LBA-6)]. Dr Mikhael pointed out that carfilzomib is favored in patients with creatinine levels ≥ 3 mg/dL or preexisting neuropathy, and pomalidomide is favored in patients who would benefit from the mode of administration or who have poorly controlled heart failure or hypertension. Combination therapy with carfilzomib and pomalidomide may be considered in patients with aggressive relapse.
Paul G. Richardson, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, USA, discussed the best timing for stem cell transplantation (SCT) for patients with MM in the era of novel therapy. Since the introduction of novel agents, the survival of patients with MM has improved significantly (P = .001) [Kumar SK et al. Leukemia. 2014]. In particular, modern induction regimens incorporating 3 drugs (specifically, IMiDs, proteasome inhibitors, and steroids) are generating ORR approaching 100% and very high rates of CR (at approximately 50%) [Richardson PG et al. Blood. 2010; Jakubowiack AJ et al. Blood. 2012]. This raises the key question—can autologous SCT (ASCT) be delayed in some transplant-eligible patients?
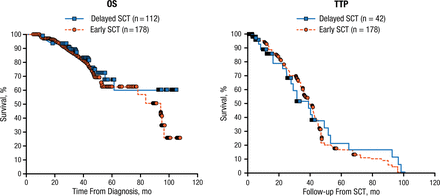
In a study that compared the outcomes of patients who underwent early or delayed SCT, 4-year OS was about 73% and 80% in both groups in patients who received immunomodulatory-drug-based/lenalidomide therapy followed by SCT or not, respectively (Figure 1) [Kumar SK et al. Cancer. 2012]. In a landmark phase 1/2 trial of lenalidomide, bortezomib, and dexamethasone (RVD), PFS was similar among patients who received ASCT or not [Richardson PG et al. Blood. 2010], a finding confirmed in a larger cohort study of over 200 patients from the group at Emory University [Nooka AK et al. Leukemia. 2014].
Effect of Timing of ASCT on Overall Survival and Time to Progression
ASCT, autologous stem cell transplantation; OS, overall survival; SCT, stem cell transplantation; TTP, time to progression.
Reproduced with permission from PG Richardson, MD.
However, Philippe Moreau, MD, University Hospital, Nantes, France, pointed out that early ASCT is feasible in about 90% of patients, whereas delayed ASCT is feasible in < 70% of patients, at least in Europe, whereas Dr Richardson argued that the availability of highly effective salvage regimens may make this a different consideration in North America. Several studies are underway to evaluate the role of timing of ASCT, including one that will randomly assign patients to undergo early ASCT, or ASCT upon relapse utilizing RVD induction, consolidation, and then lenalidomide maintenance in both arms until progression in the United States—the so-called Determination Trial, also known as CTN 1304 or DFCI/IFM 2009.
Moreover, emerging treatments for MM are continuing to change the therapeutic paradigm favorably, Dr Richardson explained. These include monoclonal antibodies in particular, such as daratumumab, which is currently under evaluation in phase 2 and 3 trials for relapsed-refractory MM, and elotuzumab, which is in phase 3 testing for the upfront treatment of MM and for relapsed-refractory MM.
Both Dr Richardson and Prof Moreau discussed some of the recent favorable data for ASCT as first-line therapy, as well as the role of consolidation and maintenance therapy, in transplant-eligible patients with MM. The use of novel agents as upfront therapy has been demonstrated to prolong OS and PFS in one phase 3 study, where patients were randomly assigned to receive high-dose melphalan plus ASCT (MEL200) or melphalan plus prednisone and lenalidomide (MPR) for consolidation, then randomly assigned to receive maintenance therapy with lenalidomide or no maintenance [Palumbo A et al. N Engl J Med. 2014]. From the start of the study to consolidation, the probability of 4-year OS was significantly greater in patients who received MEL200 compared with those who received MPR (HR, 0.55; 95% CI, 0.32 to 0.93; P = .02). The use of MEL200 plus ASCT resulted in a median PFS of 43 months and a 4-year OS of 81.6% compared with 22.4 months and 65.3%, respectively, for patients who received MPR. Whilst maintenance with lenalidomide until progression was clearly beneficial in both arms, versus placebo, the absence of a proteasome inhibitor in this study made interpretation of these results limited in the context of current treatments, where three-drug combinations have been shown to be superior, and thus these data should be considered with caution.
In conclusion, the Big 5 agents have dramatically improved PFS and OS in patients with MM. Up for debate is the timing of ASCT—whether it should remain first-line therapy in eligible patients or whether delayed transplant is best in others. Ongoing studies will hopefully provide clear answers of the most optimal timing for ASCT and the role of the novel agents in consolidation and maintenance therapy; all the speakers agreed that participation in ongoing randomized studies should be a priority.
- © 2014 SAGE Publications